Inhaled corticosteroids reduce senescence in endothelial progenitor cells from patients with COPD
- PMID: 35027472
- PMCID: PMC9120381
- DOI: 10.1136/thoraxjnl-2020-216807
Inhaled corticosteroids reduce senescence in endothelial progenitor cells from patients with COPD
Abstract
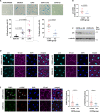
Cellular senescence contributes to the pathophysiology of chronic obstructive pulmonary disease (COPD) and cardiovascular disease. Using endothelial colony-forming-cells (ECFC), we have demonstrated accelerated senescence in smokers and patients with COPD compared with non-smokers. Subgroup analysis suggests that ECFC from patients with COPD on inhaled corticosteroids (ICS) (n=14; eight on ICS) exhibited significantly reduced senescence (Senescence-associated-beta galactosidase activity, p21CIP1), markers of DNA damage response (DDR) and IFN-γ-inducible-protein-10 compared with patients with COPD not on ICS. In vitro studies using human-umbilical-vein-endothelial-cells showed a protective effect of ICS on the DDR, senescence and apoptosis caused by oxidative stress, suggesting a protective molecular mechanism of action of corticosteroids on endothelium.
Keywords: COPD pharmacology.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: Part of this work was funded by an academic AstraZeneca AB Project Grant.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
