Neuropathogenicity of non-viable Borrelia burgdorferi ex vivo
- PMID: 35027599
- PMCID: PMC8758786
- DOI: 10.1038/s41598-021-03837-0
Neuropathogenicity of non-viable Borrelia burgdorferi ex vivo
Abstract
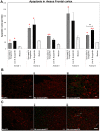
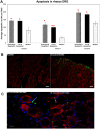
Even after appropriate treatment, a proportion of Lyme disease patients suffer from a constellation of symptoms, collectively called Post-Treatment Lyme Disease Syndrome (PTLDS). Brain PET scan of patients with PTLDS have demonstrated likely glial activation indicating persistent neuroinflammatory processes. It is possible that unresolved bacterial remnants can continue to cause neuroinflammation. In previous studies, we have shown that non-viable Borrelia burgdorferi can induce neuroinflammation and apoptosis in an oligodendrocyte cell line. In this follow-up study, we analyze the effect of sonicated remnants of B. burgdorferi on primary rhesus frontal cortex (FC) and dorsal root ganglion (DRG) explants. Five FC and three DRG tissue fragments from rhesus macaques were exposed to sonicated B. burgdorferi and analyzed for 26 inflammatory mediators. Live bacteria and medium alone served as positive and negative control, respectively. Tissues were also analyzed for cell types mediating inflammation and overall apoptotic changes. Non-viable B. burgdorferi induced significant levels of several inflammatory mediators in both FC and DRG, similar to live bacteria. However, the levels induced by non-viable B. burgdorferi was often (several fold) higher than those induced by live ones, especially for IL-6, CXCL8 and CCL2. This effect was also more profound in the FC than in the DRG. Although the levels often differed, both live and dead fragments induced the same mediators, with significant overlap between FC and DRG. In the FC, immunohistochemical staining for several inflammatory mediators showed the presence of multiple mediators in astrocytes, followed by microglia and oligodendrocytes, in response to bacterial remnants. Staining was also seen in endothelial cells. In the DRG, chemokine/cytokine staining was predominantly seen in S100 positive (glial) cells. B. burgdorferi remnants also induced significant levels of apoptosis in both the FC and DRG. Apoptosis was confined to S100 + cells in the DRG while distinct neuronal apoptosis was also detected in most FC tissues in response to sonicated bacteria. Non-viable B. burgdorferi can continue to be neuropathogenic to both CNS and PNS tissues with effects likely more profound in the former. Persistence of remnant-induced neuroinflammatory processes can lead to long term health consequences.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS, Krause PJ, Bakken JS, Strle F, Stanek G, et al. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: Clinical practice guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 2006;43:1089–1134. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

