Spatial and temporal dynamics of HDACs class IIa following mild traumatic brain injury in adult rats
- PMID: 35027678
- PMCID: PMC11629393
- DOI: 10.1038/s41380-021-01369-7
Spatial and temporal dynamics of HDACs class IIa following mild traumatic brain injury in adult rats
Abstract
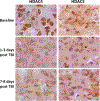
The fundamental role of epigenetic regulatory mechanisms involved in neuroplasticity and adaptive responses to traumatic brain injury (TBI) is gaining increased recognition. TBI-induced neurodegeneration is associated with several changes in the expression-activity of various epigenetic regulatory enzymes, including histone deacetylases (HDACs). In this study, PET/CT with 6-([18F]trifluoroacetamido)-1- hexanoicanilide ([18F]TFAHA) to image spatial and temporal dynamics of HDACs class IIa expression-activity in brains of adult rats subjected to a weight drop model of diffuse, non-penetrating, mild traumatic brain injury (mTBI). The mTBI model was validated by histopathological and immunohistochemical analyses of brain tissue sections for localization and magnitude of expression of heat-shock protein-70 kDa (HSP70), amyloid precursor protein (APP), cannabinoid receptor-2 (CB2), ionized calcium-binding adapter protein-1 (IBA1), histone deacetylase-4 and -5 (HDAC4 and HDAC5). In comparison to baseline, the expression-activities of HDAC4 and HDAC5 were downregulated in the hippocampus, nucleus accumbens, peri-3rd ventricular part of the thalamus, and substantia nigra at 1-3 days post mTBI, and remained low at 7-8 days post mTBI. Reduced levels of HDAC4 and HDAC5 expression observed in neurons of these brain regions post mTBI were associated with the reduced nuclear and neuropil levels of HDAC4 and HDAC5 with the shift to perinuclear localization of these enzymes. These results support the rationale for the development of therapeutic strategies to upregulate expression-activity of HDACs class IIa post-TBI. PET/CT (MRI) with [18F]TFAHA can facilitate the development and clinical translation of unique therapeutic approaches to upregulate the expression and activity of HDACs class IIa enzymes in the brain after TBI.
© 2021. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
COMPETING INTERESTS
RB and JGG are inventors on issued patents and pending patent applications related to PET imaging of HDACs class IIa expression-activity and are entitled to royalties if licensing or commercialization occurs. Potential conflicts of interest are managed in accordance with established institutional conflict of interest policies of the Wayne State University (Detroit, MI). The other authors declare that no conflicts of interest exist.
Figures

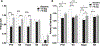


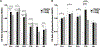
References
-
- Peterson AB, Xu L, Daugherty J, Breiding MJ. Surveillance report of traumatic brain injury-related emergency department visits, hospitalizations, and deaths. Center for Disease Control and Prevention; United States: U.S. Department of Health and Human Services, 2019.
-
- Hammond FM, Giacino JT, Nakase Richardson R, Sherer M, Zafonte RD, Whyte J, et al. Disorders of consciousness due to traumatic brain injury: functional status ten years post-injury. J Neurotrauma. 2019;36:1136–46. - PubMed
-
- Holzer KJ, Carbone JT, DeLisi M, Vaughn MG. Traumatic brain injury and coextensive psychopathology: New evidence from the 2016 Nationwide Emergency Department Sample (NEDS). J Psychiatry Res. 2019;114:149–52. - PubMed
-
- MacGregor AJ, Dougherty AL, Galarneau MR. Injury-specific correlates of combat-related traumatic brain injury in operation Iraqi freedom. J Head Trauma Rehabil. 2011;26:312–18. - PubMed
-
- Rigg JL, Mooney SR. Concussions and the military: Issues specific to service members. PMR. 2011;3:S380–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

