Anti-GD2 synergizes with CD47 blockade to mediate tumor eradication
- PMID: 35027753
- PMCID: PMC9098186
- DOI: 10.1038/s41591-021-01625-x
Anti-GD2 synergizes with CD47 blockade to mediate tumor eradication
Abstract
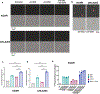
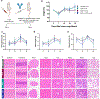
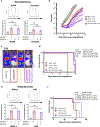
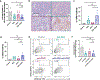
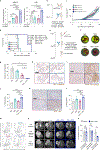
The disialoganglioside GD2 is overexpressed on several solid tumors, and monoclonal antibodies targeting GD2 have substantially improved outcomes for children with high-risk neuroblastoma. However, approximately 40% of patients with neuroblastoma still relapse, and anti-GD2 has not mediated significant clinical activity in any other GD2+ malignancy. Macrophages are important mediators of anti-tumor immunity, but tumors resist macrophage phagocytosis through expression of the checkpoint molecule CD47, a so-called 'Don't eat me' signal. In this study, we establish potent synergy for the combination of anti-GD2 and anti-CD47 in syngeneic and xenograft mouse models of neuroblastoma, where the combination eradicates tumors, as well as osteosarcoma and small-cell lung cancer, where the combination significantly reduces tumor burden and extends survival. This synergy is driven by two GD2-specific factors that reorient the balance of macrophage activity. Ligation of GD2 on tumor cells (a) causes upregulation of surface calreticulin, a pro-phagocytic 'Eat me' signal that primes cells for removal and (b) interrupts the interaction of GD2 with its newly identified ligand, the inhibitory immunoreceptor Siglec-7. This work credentials the combination of anti-GD2 and anti-CD47 for clinical translation and suggests that CD47 blockade will be most efficacious in combination with monoclonal antibodies that alter additional pro- and anti-phagocytic signals within the tumor microenvironment.
© 2022. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Competing interests
R.G.M. and C.L.M. are founders of, hold equity in and receive consulting fees from Syncopation Life Sciences. C.L.M. is a founder of, holds equity in and receives consulting fees from Lyell Immunopharma. R.G.M. and E.S. are consultants for Lyell Immunopharma. R.G.M. is a consultant for NKarta, Illumina Radiopharmaceuticals, GammaDelta Therapeutics, Aptorum Group and Zai Labs. J.T. is a consultant for Dorian Therapeutics. C.L.M. has also received consulting fees from NeoImmune Tech, Nektar Therapeutics and Apricity Health and royalties from Juno Therapeutics for CD22-CAR. W.A.W. is a founder of, holds equity in and receives consulting fees from StemSynergy Therapeutics. R.M. is on the Board of Directors of BeyondSpring and the Scientific Advisory Boards of Coherus BioSciences, Kodikaz Therapeutic Solutions and Zenshine Pharmaceuticals. R.M. and I.W. are inventors on several patents related to CD47 cancer immunotherapy that are licensed to Gilead Sciences. J.P. is an employee and shareholder of ALX Oncology, and T.C.K. and E.R.B.S. are shareholders of ALX Oncology. J.S. receives research funding from Stemcentrx/Abbvie and Pfizer and licensed a patent to Forty Seven/Gilead on the use of CD47 blocking strategies in SCLC (with I.W.). C.R.B. is a co-founder of Redwood Biosciences (a subsidiary of Catalent), Enable Biosciences, Palleon Pharmaceuticals, InterVenn Bio, Lycia Therapeutics and OliLux Biosciences and is a member of the Board of Directors of Eli Lilly. J.R.C. is a co-founder and equity holder of xCella Biosciences, Combangio and Trapeze Therapeutics. All other authors declare no competing interests.
Figures



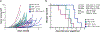






References
-
- Schulz G et al. Detection of ganglioside GD2 in tumor tissues and sera of neuroblastoma patients. Cancer Res. 44, 5914–5920 (1984). - PubMed
-
- Cheresh DA, Rosenberg J, Mujoo K, Hirschowitz L & Reisfeld RA Biosynthesis and expression of the disialoganglioside GD2, a relevant target antigen on small cell lung carcinoma for monoclonal antibody-mediated cytolysis. Cancer Res. 46, 5112–5118 (1986). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

