The preference for sugar over sweetener depends on a gut sensor cell
- PMID: 35027761
- PMCID: PMC8825280
- DOI: 10.1038/s41593-021-00982-7
The preference for sugar over sweetener depends on a gut sensor cell
Abstract
Guided by gut sensory cues, humans and animals prefer nutritive sugars over non-caloric sweeteners, but how the gut steers such preferences remains unknown. In the intestine, neuropod cells synapse with vagal neurons to convey sugar stimuli to the brain within seconds. Here, we found that cholecystokinin (CCK)-labeled duodenal neuropod cells differentiate and transduce luminal stimuli from sweeteners and sugars to the vagus nerve using sweet taste receptors and sodium glucose transporters. The two stimulus types elicited distinct neural pathways: while sweetener stimulated purinergic neurotransmission, sugar stimulated glutamatergic neurotransmission. To probe the contribution of these cells to behavior, we developed optogenetics for the gut lumen by engineering a flexible fiberoptic. We showed that preference for sugar over sweetener in mice depends on neuropod cell glutamatergic signaling. By swiftly discerning the precise identity of nutrient stimuli, gut neuropod cells serve as the entry point to guide nutritive choices.
© 2022. The Author(s).
Conflict of interest statement
Some of the findings have been filed by D.V.B. as a provisional patent application (MBF reference number 028193-9344-US01). All other authors declare no competing interests.
Figures

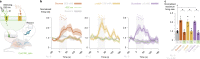

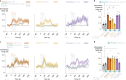


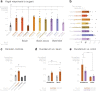

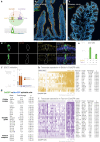






Comment in
-
Silencing gut CCK cells alters gut reaction to sugar.Nat Neurosci. 2022 Feb;25(2):136-138. doi: 10.1038/s41593-021-00998-z. Nat Neurosci. 2022. PMID: 35027762 No abstract available.
-
Neuropod cells - sensing a sweet spot in the gut.Nat Rev Gastroenterol Hepatol. 2022 Mar;19(3):147. doi: 10.1038/s41575-022-00582-1. Nat Rev Gastroenterol Hepatol. 2022. PMID: 35064255 No abstract available.
-
Sweet selection in the gut.Nat Rev Neurosci. 2022 Mar;23(3):132-133. doi: 10.1038/s41583-022-00562-z. Nat Rev Neurosci. 2022. PMID: 35102272 No abstract available.
Similar articles
-
The neural basis of sugar preference.Nat Rev Neurosci. 2022 Oct;23(10):584-595. doi: 10.1038/s41583-022-00613-5. Epub 2022 Jul 25. Nat Rev Neurosci. 2022. PMID: 35879409 Free PMC article. Review.
-
Neuropod Cells: The Emerging Biology of Gut-Brain Sensory Transduction.Annu Rev Neurosci. 2020 Jul 8;43:337-353. doi: 10.1146/annurev-neuro-091619-022657. Epub 2020 Feb 26. Annu Rev Neurosci. 2020. PMID: 32101483 Free PMC article. Review.
-
The gut-brain axis mediates sugar preference.Nature. 2020 Apr;580(7804):511-516. doi: 10.1038/s41586-020-2199-7. Epub 2020 Apr 15. Nature. 2020. PMID: 32322067 Free PMC article.
-
Differential fructose and glucose appetition in DBA/2, 129P3 and C57BL/6 × 129P3 hybrid mice revealed by sugar versus non-nutritive sweetener tests.Physiol Behav. 2021 Nov 1;241:113590. doi: 10.1016/j.physbeh.2021.113590. Epub 2021 Sep 10. Physiol Behav. 2021. PMID: 34509472
-
Evolution of complex, discreet nutrient sensing pathways.Curr Opin Clin Nutr Metab Care. 2018 Jul;21(4):289-293. doi: 10.1097/MCO.0000000000000479. Curr Opin Clin Nutr Metab Care. 2018. PMID: 29846195 Review.
Cited by
-
Dopamine drives food craving during pregnancy.Nat Metab. 2022 Apr;4(4):410-411. doi: 10.1038/s42255-022-00555-3. Nat Metab. 2022. PMID: 35379971 No abstract available.
-
Ready-to-eat food intake associates with PHQ-9-based depression in US adults: a cross-sectional study.BMC Public Health. 2025 May 13;25(1):1755. doi: 10.1186/s12889-025-22930-x. BMC Public Health. 2025. PMID: 40361049 Free PMC article.
-
The gut and heart's role in reward processing.Front Integr Neurosci. 2025 Jul 2;19:1479923. doi: 10.3389/fnint.2025.1479923. eCollection 2025. Front Integr Neurosci. 2025. PMID: 40672965 Free PMC article. Review.
-
Mechanisms and Functions of Sweet Reception in Oral and Extraoral Organs.Int J Mol Sci. 2024 Jul 5;25(13):7398. doi: 10.3390/ijms25137398. Int J Mol Sci. 2024. PMID: 39000505 Free PMC article. Review.
-
The gut microbiome in disorders of gut-brain interaction.Gut Microbes. 2024 Jan-Dec;16(1):2360233. doi: 10.1080/19490976.2024.2360233. Epub 2024 Jul 1. Gut Microbes. 2024. PMID: 38949979 Free PMC article. Review.
References
-
- Damak S, et al. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science. 2003;301:850–853. - PubMed
-
- de Araujo, I. E. et al. Food reward in the absence of taste receptor signaling. Neuron57, 930–941 (2008). - PubMed
-
- Booth DA. Satiety and behavioral caloric compensation following intragastric glucose loads in the rat. J. Comp. Physiol. Psychol. 1972;78:412–432. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

