Applications and developments of gene therapy drug delivery systems for genetic diseases
- PMID: 35027949
- PMCID: PMC8737406
- DOI: 10.1016/j.ajps.2021.05.003
Applications and developments of gene therapy drug delivery systems for genetic diseases
Abstract
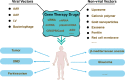
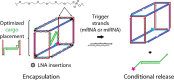
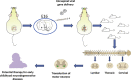
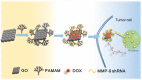
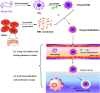
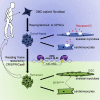
Genetic diseases seriously threaten human health and have always been one of the refractory conditions facing humanity. Currently, gene therapy drugs such as siRNA, shRNA, antisense oligonucleotide, CRISPR/Cas9 system, plasmid DNA and miRNA have shown great potential in biomedical applications. To avoid the degradation of gene therapy drugs in the body and effectively deliver them to target tissues, cells and organelles, the development of excellent drug delivery vehicles is of utmost importance. Viral vectors are the most widely used delivery vehicles for gene therapy in vivo and in vitro due to their high transfection efficiency and stable transgene expression. With the development of nanotechnology, novel nanocarriers are gradually replacing viral vectors, emerging superior performance. This review mainly illuminates the current widely used gene therapy drugs, summarizes the viral vectors and non-viral vectors that deliver gene therapy drugs, and sums up the application of gene therapy to treat genetic diseases. Additionally, the challenges and opportunities of the field are discussed from the perspective of developing an effective nano-delivery system.
Keywords: Gene therapy drugs; Genetic diseases; Nano-delivery system; Non-viral vectors; Viral vectors.
© 2021 Shenyang Pharmaceutical University. Published by Elsevier B.V.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Liu X.H., Liu M.J., Wu L.Q., Liang D.S. Gene therapy for hemophilia and duchenne muscular dystrophy in China. Hum Gene Ther. 2018;29(2):146–150. - PubMed
-
- Bhattacharyya N.P. Huntington's disease: a monogenic disorder with cellular and biochemical complexities. FEBS J. 2008;275(17):4251. - PubMed
-
- Arning L. The search for modifier genes in huntington disease multifactorial aspects of a monogenic disorder. Mol Cell Probes. 2016;30(6):404–409. - PubMed
-
- Favorova O.O., Kulakova O.G., Boiko A.N. Multiple sclerosis as a polygenic disease: an update. Russ J Genet. 2010;46(3):265–275. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

