Spectroscopic studies of the interaction between phosphorus heterocycles and cytochrome P450
- PMID: 35028181
- PMCID: PMC8740452
- DOI: 10.1016/j.jpha.2020.12.004
Spectroscopic studies of the interaction between phosphorus heterocycles and cytochrome P450
Abstract
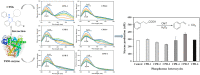
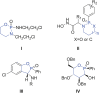
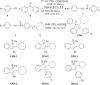
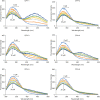
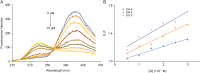
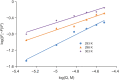
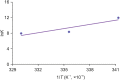
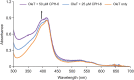
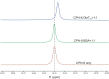
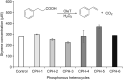
P450 fatty acid decarboxylase OleT from Staphylococcus aureus (OleTSA) is a novel cytochrome P450 enzyme that catalyzes the oxidative decarboxylation of fatty acids to yield primarily terminal alkenes and CO2 or minor α- and β-hydroxylated fatty acids as side-products. In this work, the interactions between a series of cycloalkyl phosphorus heterocycles (CPHs) and OleTSA were investigated in detail by fluorescence titration experiment, ultraviolet-visible (UV-vis) and 31P NMR spectroscopies. Fluorescence titration experiment results clearly showed that a dynamic quenching occurred when CPH-6, a representative CPHs, interacted with OleTSA with a binding constant value of 15.2 × 104 M-1 at 293 K. The thermodynamic parameters (ΔH, ΔS and ΔG) showed that the hydrogen bond and van der Waals force played major roles in the interaction between OleTSA and CPHs. The UV-vis and 31P NMR studies indicated the penetration of CPH-6 into the interior environment of OleTSA, which greatly affects the enzymatic activity of OleTSA. Therefore, our study revealed an effective way to use phosphorus heterocyclic compounds to modulate the activity of cytochrome P450 enzymes.
Keywords: Cytochrome P450; Interaction; OleT; Phosphorus heterocycles; Spectroscopy.
© 2020 Xi'an Jiaotong University. Production and hosting by Elsevier B.V.
Conflict of interest statement
The authors declare that there have no conflicts of interest.
Figures
Similar articles
-
Enhanced P450 fatty acid decarboxylase catalysis by glucose oxidase coupling and co-assembly for biofuel generation.Bioresour Technol. 2020 Sep;311:123538. doi: 10.1016/j.biortech.2020.123538. Epub 2020 May 15. Bioresour Technol. 2020. PMID: 32485602
-
Binding interaction of phosphorus heterocycles with bovine serum albumin: A biochemical study.J Pharm Anal. 2017 Feb;7(1):19-26. doi: 10.1016/j.jpha.2016.05.009. Epub 2016 Jun 15. J Pharm Anal. 2017. PMID: 29404014 Free PMC article.
-
Biochemical characterization of three new α-olefin-producing P450 fatty acid decarboxylases with a halophilic property.Biotechnol Biofuels. 2019 Apr 8;12:79. doi: 10.1186/s13068-019-1419-6. eCollection 2019. Biotechnol Biofuels. 2019. PMID: 30996734 Free PMC article.
-
Using Small Molecules to Enhance P450 OleT Enzyme Activity in Situ.Chemistry. 2021 Jun 21;27(35):8940-8945. doi: 10.1002/chem.202100680. Epub 2021 May 21. Chemistry. 2021. PMID: 33860584
-
Spectroscopic studies and molecular docking on the interaction of organotin antitumor compound bis[2,4-difluoro-N-(hydroxy-⟨κ⟩O)benzamidato-⟨κ⟩O]diphenyltin(IV) with human cytochrome P450 3A4 protease.Spectrochim Acta A Mol Biomol Spectrosc. 2016 Jun 15;163:154-61. doi: 10.1016/j.saa.2016.03.015. Epub 2016 Mar 19. Spectrochim Acta A Mol Biomol Spectrosc. 2016. PMID: 27049867
References
-
- Westheimer F.H. Why nature chose phosphates. Science. 1987;235:1173–1178. - PubMed
-
- Seto H., Kuzuyama T. Bioactive natural products with carbon-phosphorus bonds and their biosynthesis. Nat. Prod. Rep. 1999;16:589–596. - PubMed
-
- Zon G. Cyclophosphamide analogues. Prog. Med. Chem. 1982;19:205–246. - PubMed
-
- Stec W.J. Organophosphorus Chemistry, Cambridge: Royal Society of Chemistry; 1982. Cyclophosphamide and its congeners; pp. 145–174.
-
- Kafarski P., Lejczak B. Biological activity of aminophosphonic acids. Phosphorus Sulfur Silicon Relat. Elem. 1991;63:193–215.
LinkOut - more resources
Full Text Sources
Research Materials

