Drug survival of systemic immunosuppressive treatments for atopic dermatitis in a long-term pediatric cohort
- PMID: 35028369
- PMCID: PMC8714597
- DOI: 10.1016/j.ijwd.2021.07.005
Drug survival of systemic immunosuppressive treatments for atopic dermatitis in a long-term pediatric cohort
Abstract
Background: : Systemic immunosuppressive treatments are central in the treatment of severe atopic dermatitis (AD). Yet, comparative data are sparse on the performance of such immunosuppressive treatments in pediatric cohorts with severe AD.
Objective: : This study aimed to examine the drug survival of systemic immunosuppressive treatments in a cohort of children with severe AD.
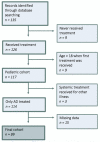
Methods: : A retrospective pediatric cohort was identified using diagnosis and treatment codes registered in medical charts. In total, 135 cases were identified; of these, 36 were excluded. All information was obtained through examination of clinical records. Drug survival was analyzed with Kaplan-Meier plots, and a log-rank test was used to test for differences in drug survival.
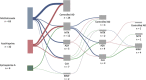
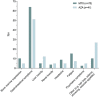
Results: : First-line treatment was primarily methotrexate (MTX; n = 63) and azathioprine (AZA; n = 32). For MTX, the drug survival rates were 69%, 50%, and 18% after 1, 2, and 4 years, respectively, with a median drug survival time of 1.58 years. For AZA, these rates were 63%, 53%, and 21%, respectively, with a median drug survival time of 1.14 years. There was no significant difference in drug survival between the treatments. The main reason for discontinuation was adverse effects (MTX: 25%; AZA: 41%). Despite this, a majority of patients experienced a good effect at the moment of discontinuation or data-lock (MTX: 60%; AZA: 53%), and treatment effect assessed as improvement in sleep quality was highly significant (p = .001). Second-line treatments included MTX (n = 12), AZA (n = 7), and cyclosporine (n = 5). These showed a median drug survival time of 1.8, 0.2, and 0.885 years, respectively.
Conclusion: : MTX and AZA were the dominant first-line treatments prescribed and were safe and equally valuable treatment options for severe childhood AD with similar drug survival outcomes. MTX was the most used second-line treatment.
Keywords: Atopic dermatitis; azathioprine; drug survival; methotrexate; pediatric; systemic immunosuppressive treatment.
© 2021 The Author(s).
Conflict of interest statement
None.
Figures

References
-
- Andersen YMF, Egeberg A, Skov L, Thyssen JP. Demographics, healthcare utilization and drug use in children and adults with atopic dermatitis in Denmark: A population-based cross-sectional study. J Eur Acad Dermatol Venereol. 2019;33(6):1133–1142. - PubMed
-
- Brunner PM, Silverberg JI, Guttman-Yassky E, Paller AS, Kabashima K, Amagai M, et al. Increasing comorbidities suggest that atopic dermatitis is a systemic disorder. J Investigat Dermatol. 2017;137(1):18–25. - PubMed
-
- Gerbens LAA, Hamann SAS, Brouwer MWD, Roekevisch E, Leeflang MMG, Spuls PI. Methotrexate and azathioprine for severe atopic dermatitis: A 5-year follow-up study of a randomized controlled trial. Br J Dermatol. 2018;178(6):1288–1296. - PubMed
LinkOut - more resources
Full Text Sources

