Impact evaluation of the efficacy of different doses of vitamin D supplementation during pregnancy on pregnancy and birth outcomes: a randomised, controlled, dose comparison trial in Pakistan
- PMID: 35028513
- PMCID: PMC8718848
- DOI: 10.1136/bmjnph-2021-000304
Impact evaluation of the efficacy of different doses of vitamin D supplementation during pregnancy on pregnancy and birth outcomes: a randomised, controlled, dose comparison trial in Pakistan
Abstract
Background: Vitamin D deficiency during pregnancy is a public health problem in Pakistan and is prevalent among most women of reproductive age in the country. Vitamin D supplementation during pregnancy is suggested to prevent adverse pregnancy outcomes and vitamin D deficiency in both the mother and her newborn.
Methods: We conducted a double-blinded, randomised controlled trial in Karachi, Pakistan to evaluate the effect of different doses of vitamin D supplementation during pregnancy on biochemical markers (serum 25(OH)D, calcium, phosphorus and alkaline phosphatase) in women and neonates, and on pregnancy and birth outcomes (gestational diabetes, pre-eclampsia, low birth weight, preterm births and stillbirths).
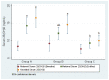
Results: Pregnant women (N=350) in their first trimester were recruited and randomised to three treatment groups of vitamin D supplementation: 4000 IU/day (group A, n=120), 2000 IU/day (group B, n=115) or 400 IU/day (group C, n=115). Women and their newborn in group A had the lowest vitamin D deficiency at endline (endline: 75.9%; neonatal: 64.9%), followed by group B (endline: 84.9%; neonatal: 73.7%) and then the control group (endline: 90.2%; neonatal: 91.8%). Vitamin D deficiency was significantly lower in group A than in group C (p=0.006) among women at endline and lower in both groups A and B than in the control group (p=0.001) in neonates. Within groups, serum 25(OH)D was significantly higher between baseline and endline in group A and between maternal baseline and neonatal levels in groups A and B. Participant serum 25(OH)D levels at the end of the trial were positively correlated with those in intervention group A (4000 IU/day) (β=4.16, 95% CI 1.6 to 6.7, p=0.002), with food group consumption (β=0.95, 95% CI 0.01 to 1.89, p=0.047) and with baseline levels of serum 25(OH)D (β=0.43, 95% CI 0.29 to 0.58, p<0.0001).
Conclusion: The evidence provided in our study indicates that vitamin D supplementation of 4000 IU/day was more effective in reducing vitamin D deficiency among pregnant women and in improving serum 25(OH)D levels in mothers and their neonates compared with 2000 IU/day and 400 IU/day. Trial registration number NCT02215213.
Keywords: nutrient deficiencies; nutrition assessment; nutritional treatment.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Regimens of vitamin D supplementation for women during pregnancy.Cochrane Database Syst Rev. 2019 Oct 3;10(10):CD013446. doi: 10.1002/14651858.CD013446. Cochrane Database Syst Rev. 2019. PMID: 31581312 Free PMC article. Review.
-
High prevalence of severe vitamin D deficiency during the first trimester in pregnant women in Switzerland and its potential contributions to adverse outcomes in the pregnancy.Swiss Med Wkly. 2020 May 28;150:w20238. doi: 10.4414/smw.2020.20238. eCollection 2020 May 18. Swiss Med Wkly. 2020. PMID: 32502277
-
The Effect of High-Dose Postpartum Maternal Vitamin D Supplementation Alone Compared with Maternal Plus Infant Vitamin D Supplementation in Breastfeeding Infants in a High-Risk Population. A Randomized Controlled Trial.Nutrients. 2019 Jul 17;11(7):1632. doi: 10.3390/nu11071632. Nutrients. 2019. PMID: 31319554 Free PMC article. Clinical Trial.
-
Efficacy of two different doses of oral vitamin D supplementation on inflammatory biomarkers and maternal and neonatal outcomes.Matern Child Nutr. 2019 Oct;15(4):e12867. doi: 10.1111/mcn.12867. Epub 2019 Sep 5. Matern Child Nutr. 2019. PMID: 31250540 Free PMC article. Clinical Trial.
-
Vitamin D supplementation for sickle cell disease.Cochrane Database Syst Rev. 2020 May 28;5(5):CD010858. doi: 10.1002/14651858.CD010858.pub3. Cochrane Database Syst Rev. 2020. PMID: 32462740 Free PMC article.
Cited by
-
An Exploration of How Solar Radiation Affects the Seasonal Variation of Human Mortality Rates and the Seasonal Variation in Some Other Common Disorders.Nutrients. 2022 Jun 17;14(12):2519. doi: 10.3390/nu14122519. Nutrients. 2022. PMID: 35745248 Free PMC article. Review.
-
Vitamin D Status and Gestational Diabetes in Russian Pregnant Women in the Period between 2012 and 2021: A Nested Case-Control Study.Nutrients. 2022 May 22;14(10):2157. doi: 10.3390/nu14102157. Nutrients. 2022. PMID: 35631298 Free PMC article.
-
Review: Influence of 25(OH)D Blood Concentration and Supplementation during Pregnancy on Preeclampsia Development and Neonatal Outcomes.Int J Mol Sci. 2022 Oct 26;23(21):12935. doi: 10.3390/ijms232112935. Int J Mol Sci. 2022. PMID: 36361738 Free PMC article. Review.
-
The Burden of Cancer, Government Strategic Policies, and Challenges in Pakistan: A Comprehensive Review.Front Nutr. 2022 Jul 22;9:940514. doi: 10.3389/fnut.2022.940514. eCollection 2022. Front Nutr. 2022. PMID: 35938114 Free PMC article. Review.
-
Long-term supplementation with 3200 to 4000 IU of vitamin D daily and adverse events: a systematic review and meta-analysis of randomized controlled trials.Eur J Nutr. 2023 Jun;62(4):1833-1844. doi: 10.1007/s00394-023-03124-w. Epub 2023 Feb 28. Eur J Nutr. 2023. PMID: 36853379 Free PMC article.
References
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
