4-Phenylbutyrate restores localization and membrane repair to human dysferlin mutations
- PMID: 35028538
- PMCID: PMC8741482
- DOI: 10.1016/j.isci.2021.103667
4-Phenylbutyrate restores localization and membrane repair to human dysferlin mutations
Abstract
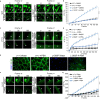
Dysferlinopathies are muscular dystrophies caused by recessive loss-of-function mutations in dysferlin (DYSF), a membrane protein involved in skeletal muscle membrane repair. We describe a cell-based assay in which human DYSF proteins bearing missense mutations are quantitatively assayed for membrane localization by flow cytometry and identified 64 localization-defective DYSF mutations. Using this platform, we show that the clinically approved drug 4-phenylbutryric acid (4-PBA) partially restores membrane localization to 25 mutations, as well as membrane repair to cultured myotubes expressing 2 different mutations. Two-day oral administration of 4-PBA to mice homozygous for one of these mutations restored myofiber membrane repair. 4-PBA may hold therapeutic potential for treating a subset of humans with muscular dystrophy due to dysferlin deficiency.
Keywords: Drugs; Musculoskeletal medicine.
© 2021 The Author(s).
Conflict of interest statement
L.P.G. is a founder of Elysium Health and Galilei Biosciences.
Figures



Similar articles
-
Proteasome inhibitors increase missense mutated dysferlin in patients with muscular dystrophy.Sci Transl Med. 2014 Aug 20;6(250):250ra112. doi: 10.1126/scitranslmed.3009612. Epub 2014 Aug 20. Sci Transl Med. 2014. Retraction in: Sci Transl Med. 2017 Jun 7;9(393):eaan8388. doi: 10.1126/scitranslmed.aan8388. PMID: 25143362 Retracted. Clinical Trial.
-
Proteasomal inhibition restores biological function of mis-sense mutated dysferlin in patient-derived muscle cells.J Biol Chem. 2012 Mar 23;287(13):10344-10354. doi: 10.1074/jbc.M111.329078. Epub 2012 Feb 8. J Biol Chem. 2012. Retraction in: J Biol Chem. 2017 Jul 28;292(30):12542. doi: 10.1074/jbc.A111.329078. PMID: 22318734 Free PMC article. Retracted.
-
Antisense oligonucleotide-mediated exon 27 skipping restores dysferlin function in dysferlinopathy patient-derived muscle cells.Mol Ther Nucleic Acids. 2024 Dec 21;36(1):102443. doi: 10.1016/j.omtn.2024.102443. eCollection 2025 Mar 11. Mol Ther Nucleic Acids. 2024. PMID: 39967852 Free PMC article.
-
Translational research and therapeutic perspectives in dysferlinopathies.Mol Med. 2011 Sep-Oct;17(9-10):875-82. doi: 10.2119/molmed.2011.00084. Epub 2011 May 6. Mol Med. 2011. PMID: 21556485 Free PMC article. Review.
-
Dysferlinopathies: Clinical and genetic variability.Clin Genet. 2022 Dec;102(6):465-473. doi: 10.1111/cge.14216. Epub 2022 Sep 6. Clin Genet. 2022. PMID: 36029111 Review.
Cited by
-
Limb Girdle Muscular Dystrophy Type 2B (LGMD2B): Diagnosis and Therapeutic Possibilities.Int J Mol Sci. 2024 May 21;25(11):5572. doi: 10.3390/ijms25115572. Int J Mol Sci. 2024. PMID: 38891760 Free PMC article. Review.
-
Cryo-EM structures of the membrane repair protein dysferlin.Nat Commun. 2024 Nov 7;15(1):9650. doi: 10.1038/s41467-024-53773-6. Nat Commun. 2024. PMID: 39511170 Free PMC article.
-
Utilization of Targeted RNA-Seq for the Resolution of Variant Pathogenicity and Enhancement of Diagnostic Yield in Dysferlinopathy.J Pers Med. 2023 Mar 13;13(3):520. doi: 10.3390/jpm13030520. J Pers Med. 2023. PMID: 36983702 Free PMC article.
-
4-phenylbutyric acid re-trafficking hERG/G572R channel protein by modulating the endoplasmic reticulum stress-associated chaperones and endoplasmic reticulum-associated degradation gene.J Thorac Dis. 2023 Aug 31;15(8):4472-4485. doi: 10.21037/jtd-23-1252. Epub 2023 Aug 28. J Thorac Dis. 2023. PMID: 37691654 Free PMC article.
-
Redefining the architecture of ferlin proteins: Insights into multi-domain protein structure and function.PLoS One. 2022 Jul 28;17(7):e0270188. doi: 10.1371/journal.pone.0270188. eCollection 2022. PLoS One. 2022. PMID: 35901179 Free PMC article.
References
-
- Bersch K. Georg-August-University Göttingen; 2017. Dysferlin in Skeletal and Heart Muscle: From Trafficking to Therapy.
-
- Cacciottolo M., Numitone G., Aurino S., Caserta I.R., Fanin M., Politano L., Minetti C., Ricci E., Piluso G., Angelini C., Nigro V. Muscular dystrophy with marked Dysferlin deficiency is consistently caused by primary dysferlin gene mutations. Eur. J. Hum. Genet. 2011;19:974–980. doi: 10.1038/ejhg.2011.70. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

