Role of von Willebrand factor in venous thromboembolic disease
- PMID: 35028601
- PMCID: PMC8739873
- DOI: 10.1016/j.jvssci.2021.08.002
Role of von Willebrand factor in venous thromboembolic disease
Abstract
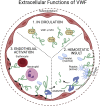
Objective: Evolving evidence of the shared risk factors and pathogenic mechanisms in arterial and venous thrombosis questions of the strict vascular dichotomy of arterial vs venous. The connection between arterial and venous thrombosis has been highlighted by common underlying inflammatory processes, a concept known as thromboinflammatory disease. Using this relationship, we can apply knowledge from arterial disease to better understand and potentially mitigate venous disease. A protein that has been extensively studied in atherothrombotic disease and inflammation is von Willebrand factor (VWF). Because many predisposing and provoking factors of venous thromboembolism (VTE) have been shown to directly modulate VWF levels, it is, perhaps, not surprising that VWF has been highlighted by several recent association studies of patients with VTE.
Methods: In the present narrative review, we investigated more deeply the effects of VWF in venous disease by synthesizing the data from clinical studies of deep vein thrombosis of the limbs, pulmonary embolism, portal and cerebral vein thrombosis, and the complications of thrombosis, including post-thrombotic syndrome, venous insufficiency, and chronic thromboembolic pulmonary hypertension. We have also discussed the findings from preclinical studies to highlight novel VWF biochemistry in thrombosis and therapeutics.
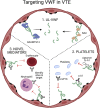
Results: Across the spectrum of venous thromboembolic disease, we consistently observed that elevated VWF levels conferred an increased risk of VTE and long-term venous complications. We have highlighted important findings from VWF molecular research and have proposed mechanisms by which VWF participates in venous disease. Emerging evidence from preclinical studies might reveal novel targets for thromboinflammatory disease, including specific VWF pathophysiology. Furthermore, we have highlighted the utility of measuring VWF to prognosticate and risk stratify for VTE and its complications.
Conclusions: As the prevalence of inflammatory processes, such as aging, obesity, and diabetes increases in our population, it is critical to understand the evolving role of VWF in venous disease to guide clinical decisions and therapeutics.
Keywords: Thromboinflammatory; Venous disease; Venous thromboembolism; von Willebrand factor.
© 2021 by the Society for Vascular Surgery. Published by Elsevier Inc.
Figures


References
-
- Raskob G.E., Angchaisuksiri P., Blanco A.N., Buller H., Gallus A., Hunt B.J., et al. Thrombosis: a major contributor to global disease burden. Arterioscler Thromb Vasc Biol. 2014;34:2363–2371. - PubMed
-
- Tan V.P., Chung A., Yan B.P., Gibson P.R. Venous and arterial disease in inflammatory bowel disease. J Gastroenterol Hepatol. 2013;28:1095–1113. - PubMed
-
- Franchini M., Mannucci P.M. Venous and arterial thrombosis: different sides of the same coin? Eur J Intern Med. 2008;19:476–481. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

