Extracellular vesicle-mediated delivery of circDYM alleviates CUS-induced depressive-like behaviours
- PMID: 35029057
- PMCID: PMC8758833
- DOI: 10.1002/jev2.12185
Extracellular vesicle-mediated delivery of circDYM alleviates CUS-induced depressive-like behaviours
Abstract
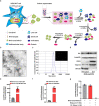
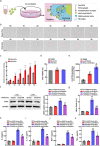
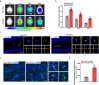
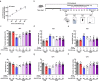
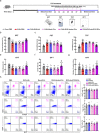
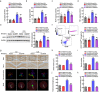
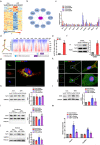
Major depressive disorder (MDD) is the most prevalent psychiatric disorder worldwide and severely limits psychosocial function and quality of life, but no effective medication is currently available. Circular RNAs (circRNAs) have been revealed to participate in the MDD pathological process. Targeted delivery of circRNAs without blood-brain barrier (BBB) restriction for remission of MDD represents a promising approach for antidepressant therapy. In this study, RVG-circDYM-extracellular vesicles (RVG-circDYM-EVs) were engineered to target and preferentially transfer circDYM to the brain, and the effect on the pathological process in a chronic unpredictable stress (CUS) mouse model of depression was investigated. The results showed that RVG-circDYM-EVs were successfully purified by ultracentrifugation from overexpressed circDYM HEK 293T cells, and the characterization of RVG-circDYM-EVs was successfully demonstrated in terms of size, morphology and specific markers. Beyond demonstrating proof-of-concept for an RNA drug delivery technology, we observed that systemic administration of RVG-circDYM-EVs efficiently delivered circDYM to the brain, and alleviated CUS-induced depressive-like behaviours, and we discovered that RVG-circDYM-EVs notably inhibited microglial activation, BBB leakiness and peripheral immune cells infiltration, and attenuated astrocyte disfunction induced by CUS. CircDYM can bind mechanistically to the transcription factor TAF1 (TATA-box binding protein associated factor 1), resulting in the decreased expression of its downstream target genes with consequently suppressed neuroinflammation. Taken together, our findings suggest that extracellular vesicle-mediated delivery of circDYM is effective for MDD treatment and promising for clinical applications.
Keywords: CircDYM; MDD; TAF1; extracellular vesicles; inflammation.
© 2022 The Authors. Journal of Extracellular Vesicles published by Wiley Periodicals, LLC on behalf of the International Society for Extracellular Vesicles.
Conflict of interest statement
The author declared no conflict of interest.
Figures


References
-
- Abbott, N. J. , Patabendige, A. A. K. , Dolman, D. E. M. , Yusof, S. R. , & Begley, D. J. (2010). Structure and function of the blood‐brain barrier. Neurobiology of Disease, 37, 13–25. - PubMed
-
- Alvarez‐Erviti, L. , Seow, Y. , Yin, H. , Betts, C. , Lakhal, S. , & Wood, M. J. A. (2011). Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nature Biotechnology, 29, 341–345. - PubMed
-
- Arnow, B. A. , Blasey, C. , Williams, L. M. , Palmer, D. M. , Rekshan, W. , Schatzberg, A. F. , Etkin, A. , Kulkarni, J. , Luther, J. F. , & Rush, A. J. (2015). Depression subtypes in predicting antidepressant response: A report from the iSPOT‐D trial. American Journal of Psychiatry, 172, 743–750. - PubMed
-
- Athauda, D. , Gulyani, S. , Karnati, H. K. , Li, Y. , Tweedie, D. , Mustapic, M. , Chawla, S. , Chowdhury, K. , Skene, S. S. , Greig, N. H. , Kapogiannis, D. , & Foltynie, T. (2019). Utility of neuronal‐derived exosomes to examine molecular mechanisms that affect motor function in patients with Parkinson disease: A secondary analysis of the exenatide‐PD trial. JAMA Neurology, 76, 420–429. - PMC - PubMed
-
- Belzeaux, R. , Gorgievski, V. , Fiori, L. M. , Lopez, J. P. , Grenier, J. , Lin, R. , Nagy, C. , Ibrahim, ElC , Gascon, E. , Courtet, P. , Richard‐Devantoy, S. , Berlim, M. , Chachamovich, E. , Théroux, J. ‐F. , Dumas, S. , Giros, B. , Rotzinger, S. , Soares, C. N. , Foster, J. A. , … Turecki, G. (2020). GPR56/ADGRG1 is associated with response to antidepressant treatment. Nature Communication, 11, 1635. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

