Human neural cell type-specific extracellular vesicle proteome defines disease-related molecules associated with activated astrocytes in Alzheimer's disease brain
- PMID: 35029059
- PMCID: PMC8758831
- DOI: 10.1002/jev2.12183
Human neural cell type-specific extracellular vesicle proteome defines disease-related molecules associated with activated astrocytes in Alzheimer's disease brain
Abstract
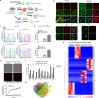
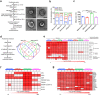
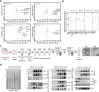
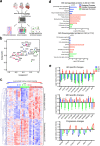
In neurodegenerative diseases, extracellular vesicles (EVs) transfer pathogenic molecules and are consequently involved in disease progression. We have investigated the proteomic profiles of EVs that were isolated from four different human-induced pluripotent stem cell-derived neural cell types (excitatory neurons, astrocytes, microglia-like cells, and oligodendrocyte-like cells). Novel cell type-specific EV protein markers were then identified for the excitatory neurons (ATP1A3, NCAM1), astrocytes (LRP1, ITGA6), microglia-like cells (ITGAM, LCP1), and oligodendrocyte-like cells (LAMP2, FTH1), as well as 16 pan-EV marker candidates, including integrins and annexins. To further demonstrate how cell-type-specific EVs may be involved in Alzheimer's disease (AD), we performed protein co-expression network analysis and conducted cell type assessments for the proteomes of brain-derived EVs from the control, mild cognitive impairment, and AD cases. A protein module enriched in astrocyte-specific EV markers was most significantly associated with the AD pathology and cognitive impairment, suggesting an important role in AD progression. The hub protein from this module, integrin-β1 (ITGB1), was found to be significantly elevated in astrocyte-specific EVs enriched from the total brain-derived AD EVs and associated with the brain β-amyloid and tau load in independent cohorts. Thus, our study provides a featured framework and rich resource for the future analyses of EV functions in neurodegenerative diseases in a cell type-specific manner.
© 2021 The Authors. Journal of Extracellular Vesicles published by Wiley Periodicals, LLC on behalf of the International Society for Extracellular Vesicles.
Conflict of interest statement
T.I. has a sponsored research agreement from Abbvie, Inc. for this study. M.B.J. and W.W.P. are co‐inventors of patent WO/2018/160496, related to the differentiation of human pluripotent stem cells into microglia.
Figures



References
-
- Abud, E. M. , Ramirez, R. N. , Martinez, E. S. , Healy, L. M. , Nguyen, C. H. H. , Newman, S. A. , Yeromin, A. V. , Scarfone, V. M. , Marsh, S. E. , Fimbres, C. , Caraway, C. A. , Fote, G. M. , Madany, A. M. , Agrawal, A. , Kayed, R. , Gylys, K. H. , Cahalan, M. D. , Cummings, B. J. , Antel, J. P. , … Blurton‐Jones, M. (2017). iPSC‐derived human microglia‐like cells to study neurological diseases. Neuron, 94, 278–293 e279. 10.1016/j.neuron.2017.03.042 - DOI - PMC - PubMed
-
- Barbar, L. , Jain, T. , Zimmer, M. , Kruglikov, I. , Sadick, J. S. , Wang, M. , Kalpana, K. , Rose, I. V. L. , Burstein, S. R. , Rusielewicz, T. , Nijsure, M. , Guttenplan, K. A. , Di Domenico, A. , Croft, G. , Zhang, B. , Nobuta, H. , Hébert, J. M. , Liddelow, S. A. , & Fossati, V. (2020). CD49f is a novel marker of functional and reactive human iPSC‐derived astrocytes. Neuron, 107, 436–453 e412. 10.1016/j.neuron.2020.05.014 - DOI - PMC - PubMed
-
- Brennan, K. , Martin, K. , FitzGerald, S. P. , O'Sullivan, J. , Wu, Y. , Blanco, A. , Richardson, C. , & Mc Gee, M. M. (2020). A comparison of methods for the isolation and separation of extracellular vesicles from protein and lipid particles in human serum. Scientific Reports, 10, 1039 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

