Safety of the BNT162b2 mRNA COVID-19 vaccine in oncologic patients undergoing numerous cancer treatment options: A retrospective single-center study
- PMID: 35029223
- PMCID: PMC8758044
- DOI: 10.1097/MD.0000000000028561
Safety of the BNT162b2 mRNA COVID-19 vaccine in oncologic patients undergoing numerous cancer treatment options: A retrospective single-center study
Abstract
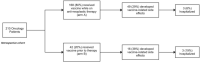
The COVID-19 pandemic, caused by the SARS-CoV2 virus, has infected millions worldwide with cancer patients demonstrating a higher prevalence for severe disease and poorer outcomes. Recently, the BNT162b2 mRNA COVID-19 vaccine was released as the primary means to combat COVID-19. The currently reported incidence of local and systemic side effects was 27% in the general public. The safety of the BNT162b2 mRNA COVID-19 vaccine has not been studied in patients with an active cancer diagnosis who are either ongoing or plan to undergo oncologic therapy.This single center study reviewed the charts of 210 patients with active cancer diagnoses that received both doses of the BNT162b2 mRNA COVID-19 vaccine. The development of side effects from the vaccine, hospitalizations or exacerbations from various oncologic treatment were documented. Type of oncologic treatment (immunotherapy, chemotherapy, hormonal, biologic, radiation or mixed) was documented to identify if side effects were related to treatment type. The time at which the vaccine was administered in relation to treatment onset (on long term therapy, within 1 month of therapy or prior to therapy) was also documented to identify any relationships.Sixty five (31%) participants experienced side effects from the BNT162b2 mRNA COVID-19 vaccine, however most were mild to moderate. Treatment protocol was not linked to the development of vaccine related side effects (P = .202), nor was immunotherapy (P = .942). The timing of vaccine administered in relation to treatment onset was also not related to vaccine related side effects (P = .653). Six (2.9%) participants were hospitalized and 4 (2%) died.The incidence of side effects in cancer patients is similar to what has been reported for the general public (31% vs 27%). Therefore, we believe that the BNT162b2 mRNA COVID-19 vaccine is safe in oncologic patients undergoing numerous cancer treatments.
Copyright © 2022 the Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors have no funding and conflicts of interests to disclose.
Figures
Similar articles
-
Immunogenicity and safety of a booster dose of a self-amplifying RNA COVID-19 vaccine (ARCT-154) versus BNT162b2 mRNA COVID-19 vaccine: a double-blind, multicentre, randomised, controlled, phase 3, non-inferiority trial.Lancet Infect Dis. 2024 Apr;24(4):351-360. doi: 10.1016/S1473-3099(23)00650-3. Epub 2023 Dec 20. Lancet Infect Dis. 2024. PMID: 38141632 Clinical Trial.
-
Predictors of poor seroconversion and adverse events to SARS-CoV-2 mRNA BNT162b2 vaccine in cancer patients on active treatment.Eur J Cancer. 2021 Dec;159:105-112. doi: 10.1016/j.ejca.2021.09.030. Epub 2021 Oct 11. Eur J Cancer. 2021. PMID: 34742157 Free PMC article.
-
Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine.N Engl J Med. 2020 Dec 31;383(27):2603-2615. doi: 10.1056/NEJMoa2034577. Epub 2020 Dec 10. N Engl J Med. 2020. PMID: 33301246 Free PMC article. Clinical Trial.
-
Review of COVID-19 mRNA Vaccines: BNT162b2 and mRNA-1273.J Pharm Pract. 2022 Dec;35(6):947-951. doi: 10.1177/08971900211009650. Epub 2021 Apr 12. J Pharm Pract. 2022. PMID: 33840294 Review.
-
Psoriasis and biological drugs at the time of SARS-CoV-2 infection: a mini review outlining risk of infection, seroprevalence, and safety and efficacy of the BNT162b2 vaccine.Front Immunol. 2024 Jan 30;15:1354729. doi: 10.3389/fimmu.2024.1354729. eCollection 2024. Front Immunol. 2024. PMID: 38352875 Free PMC article. Review.
Cited by
-
Safety of COVID-19 Vaccine in Patients with Cancer in a High-Volume Comprehensive Cancer Center.Oncologist. 2022 Mar 4;27(2):e203-e205. doi: 10.1093/oncolo/oyab037. Oncologist. 2022. PMID: 35641217 Free PMC article.
-
Immunogenicity and adverse events of the COVID-19 vaccines in healthy and individuals with autoimmune diseases in an Iranian population.Int J Immunopathol Pharmacol. 2024 Jan-Dec;38:3946320241239202. doi: 10.1177/03946320241239202. Int J Immunopathol Pharmacol. 2024. PMID: 38494849 Free PMC article.
-
SARS-CoV-2 infection and COVID-19 vaccination in cancer patients undergoing immune checkpoint inhibitors.Cell Death Dis. 2023 Jun 30;14(6):390. doi: 10.1038/s41419-023-05922-w. Cell Death Dis. 2023. PMID: 37391394 Free PMC article. Review.
-
Impact of chemotherapy and/or immunotherapy on neutralizing antibody response to SARS-CoV-2 mRNA-1237 vaccine in patients with solid tumors.Mol Oncol. 2023 Apr;17(4):686-694. doi: 10.1002/1878-0261.13359. Epub 2022 Dec 30. Mol Oncol. 2023. PMID: 36495129 Free PMC article.
-
Cancer Patients and the COVID-19 Vaccines: Considerations and Challenges.Cancers (Basel). 2022 Nov 16;14(22):5630. doi: 10.3390/cancers14225630. Cancers (Basel). 2022. PMID: 36428722 Free PMC article. Review.
References
-
- WHO Coronavirus Disease (COVID-19) Dashboard | WHO Coronavirus Disease (COVID-19) Dashboard [Internet]. Available from: https://covid19.who.int/. Accessed January 15, 2021.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous