Understanding Nanomaterial-Liver Interactions to Facilitate the Development of Safer Nanoapplications
- PMID: 35029313
- PMCID: PMC9040585
- DOI: 10.1002/adma.202106456
Understanding Nanomaterial-Liver Interactions to Facilitate the Development of Safer Nanoapplications
Abstract
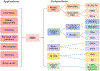
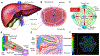
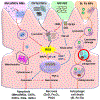
Nanomaterials (NMs) are widely used in commercial and medical products, such as cosmetics, vaccines, and drug carriers. Exposure to NMs via various routes such as dermal, inhalation, and ingestion has been shown to gain access to the systemic circulation, resulting in the accumulation of NMs in the liver. The unique organ structures and blood flow features facilitate the liver sequestration of NMs, which may cause adverse effects in the liver. Currently, most in vivo studies are focused on NMs accumulation at the organ level and evaluation of the gross changes in liver structure and functions, however, cell-type-specific uptake and responses, as well as the molecular mechanisms at cellular levels leading to effects at organ levels are lagging. Herein, the authors systematically review diverse interactions of NMs with the liver, specifically on major liver cell types including Kupffer cells (KCs), liver sinusoidal endothelial cells (LSECs), hepatic stellate cells (HSCs), and hepatocytes as well as the detailed molecular mechanisms involved. In addition, the knowledge gained on nano-liver interactions that can facilitate the development of safer nanoproducts and nanomedicine is also reviewed.
Keywords: cell-type-specific uptake; mode of action; nano-liver interaction; nanomedicine; nanosafety.
© 2022 Wiley-VCH GmbH.
Figures





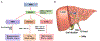



References
-
- Baig N, Kammakakam I, Falath W, Materials Advances 2021, 2, 1821.
-
- Smith BR, Gambhir SS, Chem. Rev 2017, 117, 901. - PubMed
-
- Valsami-Jones E, Lynch I, Science 2015, 350, 388. - PubMed
-
- Hochella M, Mogk DW, Ranville J, Allen IC, Luther GW, Marr LC, Mcgrail BP, Murayama M, Qafoku NP, Rosso KM, Science 2019, 363, 1414. - PubMed
-
- Khin MM, Nair AS, Babu VJ, Murugan R, Ramakrishna S, Energ. Environ. Sci 2012, 5, 8075.

