Effect of Subcutaneous Casirivimab and Imdevimab Antibody Combination vs Placebo on Development of Symptomatic COVID-19 in Early Asymptomatic SARS-CoV-2 Infection: A Randomized Clinical Trial
- PMID: 35029629
- PMCID: PMC8808333
- DOI: 10.1001/jama.2021.24939
Effect of Subcutaneous Casirivimab and Imdevimab Antibody Combination vs Placebo on Development of Symptomatic COVID-19 in Early Asymptomatic SARS-CoV-2 Infection: A Randomized Clinical Trial
Erratum in
-
Collaborator Name Misspelled.JAMA. 2022 May 24;327(20):2024. doi: 10.1001/jama.2022.7596. JAMA. 2022. PMID: 35608600 Free PMC article. No abstract available.
Abstract
Importance: Easy-to-administer anti-SARS-CoV-2 treatments may be used to prevent progression from asymptomatic infection to symptomatic disease and to reduce viral carriage.
Objective: To evaluate the effect of combination subcutaneous casirivimab and imdevimab on progression from early asymptomatic SARS-CoV-2 infection to symptomatic COVID-19.
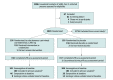
Design, setting, and participants: Randomized, double-blind, placebo-controlled, phase 3 trial of close household contacts of a SARS-CoV-2-infected index case at 112 sites in the US, Romania, and Moldova enrolled July 13, 2020-January 28, 2021; follow-up ended March 11, 2021. Asymptomatic individuals (aged ≥12 years) were eligible if identified within 96 hours of index case positive test collection. Results from 314 individuals positive on SARS-CoV-2 reverse transcriptase-quantitative polymerase chain reaction (RT-qPCR) testing are reported.
Interventions: Individuals were randomized 1:1 to receive 1 dose of subcutaneous casirivimab and imdevimab, 1200 mg (600 mg of each; n = 158), or placebo (n = 156).
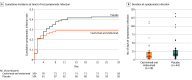
Main outcomes and measures: The primary end point was the proportion of seronegative participants who developed symptomatic COVID-19 during the 28-day efficacy assessment period. The key secondary efficacy end points were the number of weeks of symptomatic SARS-CoV-2 infection and the number of weeks of high viral load (>4 log10 copies/mL).
Results: Among 314 randomized participants (mean age, 41.0 years; 51.6% women), 310 (99.7%) completed the efficacy assessment period; 204 were asymptomatic and seronegative at baseline and included in the primary efficacy analysis. Subcutaneous casirivimab and imdevimab, 1200 mg, significantly prevented progression to symptomatic disease (29/100 [29.0%] vs 44/104 [42.3%] with placebo; odds ratio, 0.54 [95% CI, 0.30-0.97]; P = .04; absolute risk difference, -13.3% [95% CI, -26.3% to -0.3%]). Casirivimab and imdevimab reduced the number of symptomatic weeks per 1000 participants (895.7 weeks vs 1637.4 weeks with placebo; P = .03), an approximately 5.6-day reduction in symptom duration per symptomatic participant. Treatment with casirivimab and imdevimab also reduced the number of high viral load weeks per 1000 participants (489.8 weeks vs 811.9 weeks with placebo; P = .001). The proportion of participants receiving casirivimab and imdevimab who had 1 or more treatment-emergent adverse event was 33.5% vs 48.1% for placebo, including events related (25.8% vs 39.7%) or not related (11.0% vs 16.0%) to COVID-19.
Conclusions and relevance: Among asymptomatic SARS-CoV-2 RT-qPCR-positive individuals living with an infected household contact, treatment with subcutaneous casirivimab and imdevimab antibody combination vs placebo significantly reduced the incidence of symptomatic COVID-19 over 28 days.
Trial registration: ClinicalTrials.gov Identifier: NCT04452318.
Conflict of interest statement
Figures
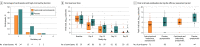
Update of
-
Subcutaneous REGEN-COV Antibody Combination in Early Asymptomatic SARS-CoV-2 Infection: A Randomized Clinical Trial.medRxiv [Preprint]. 2021 Sep 18:2021.06.14.21258569. doi: 10.1101/2021.06.14.21258569. medRxiv. 2021. Update in: JAMA. 2022 Feb 1;327(5):432-441. doi: 10.1001/jama.2021.24939. PMID: 34159343 Free PMC article. Updated. Preprint.
Comment in
-
Realizing the Potential of Anti-SARS-CoV-2 Monoclonal Antibodies for COVID-19 Management.JAMA. 2022 Feb 1;327(5):427-429. doi: 10.1001/jama.2021.19994. JAMA. 2022. PMID: 35029644 No abstract available.
References
-
- Centers for Disease Control and Prevention . Clinical Questions About COVID-19: Questions and Answers. Accessed April 23, 2021. https://www.cdc.gov/coronavirus/2019-ncov/hcp/faq.html#Transmission
-
- GISAID . hCov19 Variants. Accessed July 20, 2021. https://www.gisaid.org/hcov19-variants/
-
- Higdon MM, Wahl B, Jones CB, et al. . A systematic review of COVID-19 vaccine efficacy and effectiveness against SARS-CoV-2 infection and disease. medRxiv. Preprint posted September 25, 2021. doi:10.1101/2021.09.17.21263549 - DOI
-
- World Health Organization . Coronavirus (COVID-19) dashboard. Accessed November 2, 2021. https://covid19.who.int/table
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- K12 HL143886/HL/NHLBI NIH HHS/United States
- UM1 AI069534/AI/NIAID NIH HHS/United States
- P30 AI045008/AI/NIAID NIH HHS/United States
- UM1 AI069481/AI/NIAID NIH HHS/United States
- UM1 AI148684/AI/NIAID NIH HHS/United States
- UM1 AI154468/AI/NIAID NIH HHS/United States
- UM1 AI148576/AI/NIAID NIH HHS/United States
- U01 HL157004/HL/NHLBI NIH HHS/United States
- UM1 AI068635/AI/NIAID NIH HHS/United States
- UM1 AI068619/AI/NIAID NIH HHS/United States
- UL1 TR002544/TR/NCATS NIH HHS/United States
- UM1 AI068617/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

