Development of a sample preparation procedure for Sr isotope analysis of Portland cements
- PMID: 35029692
- PMCID: PMC9142479
- DOI: 10.1007/s00216-021-03821-7
Development of a sample preparation procedure for Sr isotope analysis of Portland cements
Abstract
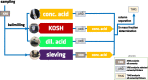
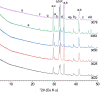
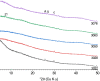
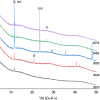
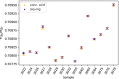
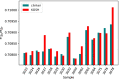
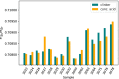
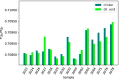
The 87Sr/86Sr isotope ratio can, in principle, be used for provenancing of cement. However, while commercial cements consist of multiple components, no detailed investigation into their individual 87Sr/86Sr isotope ratios or their influence on the integral 87Sr/86Sr isotope ratio of the resulting cement was conducted previously. Therefore, the present study aimed at determining and comparing the conventional 87Sr/86Sr isotope ratios of a diverse set of Portland cements and their corresponding Portland clinkers, the major component of these cements. Two approaches to remove the additives from the cements, i.e. to measure the conventional 87Sr/86Sr isotopic fingerprint of the clinker only, were tested, namely, treatment with a potassium hydroxide/sucrose solution and sieving on a 11-µm sieve. Dissolution in concentrated hydrochloric acid/nitric acid and in diluted nitric acid was employed to determine the 87Sr/86Sr isotope ratios of the cements and the individual clinkers. The aim was to find the most appropriate sample preparation procedure for cement provenancing, and the selection was realised by comparing the 87Sr/86Sr isotope ratios of differently treated cements with those of the corresponding clinkers. None of the methods to separate the clinkers from the cements proved to be satisfactory. However, it was found that the 87Sr/86Sr isotope ratios of clinker and cement generally corresponded, meaning that the latter can be used as a proxy for the clinker 87Sr/86Sr isotope ratio. Finally, the concentrated hydrochloric acid/nitric acid dissolution method was found to be the most suitable sample preparation method for the cements; it is thus recommended for 87Sr/86Sr isotope analyses for cement provenancing.
Keywords: Cement; Clinker; Provenancing; Sample preparation; Strontium isotopes.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Taylor HFW. Cement chemistry. 2. London: Thomas Telford Ltd.; 2009. p. 459.
-
- Kazlagic A, Vogl J, Gluth GJG, Stephan D. Provenancing of cement using elemental analyses and isotope techniques – the state-of-the-art and future perspectives. J Anal At Spectrom. 2021;36:2030–2042. doi: 10.1039/D1JA00144B. - DOI
-
- Goguel RL, Stjohn DA. Chemical-identification of Portland cements in New-Zealand concretes .1. Characteristic differences among New-Zealand cements in minor and trace-element chemistry. Cem Concr Res. 1993;23(1):59–68. doi: 10.1016/0008-8846(93)90136-W. - DOI
-
- Potgieter JH, Potgieter SS, McCrindle RI, Tamas FD. Fingerprinting of South African cement clinkers and gypsum as a tool for cement identification purposes. Adv Cem Res. 2003;15(2):45–50. doi: 10.1680/adcr.15.2.45.36725. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

