Ibrutinib in Combination With Rituximab for Indolent Clinical Forms of Mantle Cell Lymphoma (IMCL-2015): A Multicenter, Open-Label, Single-Arm, Phase II Trial
- PMID: 35030036
- PMCID: PMC8987223
- DOI: 10.1200/JCO.21.02321
Ibrutinib in Combination With Rituximab for Indolent Clinical Forms of Mantle Cell Lymphoma (IMCL-2015): A Multicenter, Open-Label, Single-Arm, Phase II Trial
Abstract
Purpose: The need for an individualized management of indolent clinical forms in mantle cell lymphoma (MCL) is increasingly recognized. We hypothesized that a tailored treatment with ibrutinib in combination with rituximab (IR) could obtain significant responses in these patients.
Methods: This is a multicenter single-arm, open-label, phase II study with a two-stage design conducted in 12 Spanish GELTAMO sites (ClinicalTrials.gov identifier: NCT02682641). Previously untreated MCL patients with indolent clinical forms defined by the following criteria were eligible: no disease-related symptoms, nonblastoid variants, Ki-67 < 30%, and largest tumor diameter ≤ 3 cm. Both leukemic non-nodal and nodal subtypes were recruited. Patients received ibrutinib 560 mg once daily and a total of eight doses of rituximab 375 mg/m2. Ibrutinib could be discontinued after 2 years in the case of sustained undetectable minimal residual disease (MRD). The primary end point was the complete response (CR) rate achieved after 12 cycles according to Lugano criteria.
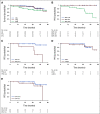
Results: Fifty patients with MCL (male 66%; median age 65 years) were enrolled. After 12 cycles of treatment, 42 (84%; 95% CI, 74 to 94) patients had an overall response, including 40 (80%; 95% CI, 69 to 91) with CR. Moreover, undetectable MRD in peripheral blood was achieved in 87% (95% CI, 77 to 97) of cases. At 2 years, 24 of 35 evaluable patients (69%) could discontinue ibrutinib because of undetectable MRD. Four patients had disease progression; three were non-nodal MCL and carried high genomic complexity and TP53 mutations at enrollment. No unexpected toxicity was seen except one patient with severe aplastic anemia.
Conclusion: Frontline IR combination achieves a high rate of CRs and undetectable MRD in indolent clinical forms of MCL. Discontinuation seems appropriate in cases with undetectable MRD, except for TP53-mutated cases.
Conflict of interest statement
Figures
References
-
- Swerdlow SH, Campo E, Seto M, et al. in Swerdlow SH, Campo E, Harris NL, et al (eds): WHO Classification of Tumors of Hematopoietic and Lymphoid Tissues (rev ed 4). Lyon, France, IARC. 2017. Mantle cell lymphoma; pp. 285–290.
-
- Orchard J, Garand R, Davis Z, et al. A subset of t(11;14) lymphoma with mantle cell features displays mutated IgVH genes and includes patients with good prognosis, nonnodal disease. Blood. 2003;101:4975–4981. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

