Antibodies to watch in 2022
- PMID: 35030985
- PMCID: PMC8765076
- DOI: 10.1080/19420862.2021.2014296
Antibodies to watch in 2022
Abstract
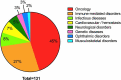
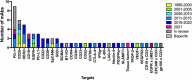
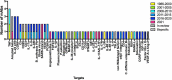
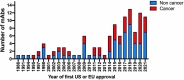
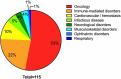
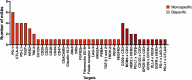
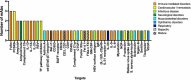
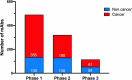
In this 13th annual installment of the annual 'Antibodies to Watch' article series, we discuss key events in commercial antibody therapeutics development that occurred in 2021 and forecast events that might occur in 2022. Regulatory review of antibody therapeutics that target the SARS-CoV-2 coronavirus proceeded at an unprecedented pace in 2021, resulting in both emergency use authorizations and full approvals for sotrovimab, regdanvimab, REGEN-COV2, as well as others, in numerous countries. As of November 1, a total of 11 antibody therapeutics had been granted first approvals in either the United States or European Union in 2021 (evinacumab, dostarlimab loncastuximab tesirine, amivantamab, aducanumab, tralokinumab, anifrolumab, bimekizumab, tisotumab vedotin, regdanvimab, REGEN-COV2). The first global approvals of seven products, however, were granted elsewhere, including Japan (pabinafusp alfa), China (disitamab vedotin, penpulimab, zimberelimab), Australia (sotrovimab, REGEN-COV2), or the Republic of Korea (regdanvimab). Globally, at least 27 novel antibody therapeutics are undergoing review by regulatory agencies. First actions by the Food and Drug Administration on the biologics license applications for faricimab, sutimlimab, tebentafusp, relatlimab, sintilimab, ublituximab and tezepelumab are expected in the first quarter of 2022. Finally, our data show that, with antibodies for COVID-19 excluded, the late-stage commercial clinical pipeline of antibody therapeutics grew by over 30% in the past year. Of those in late-stage development, marketing applications for at least 22 may occur by the end of 2022.
Keywords: COVID-19; European Medicines Agency; Food and Drug Administration; SARS-CoV-2; antibody therapeutics; cancer; immune-mediated disorders.
Conflict of interest statement
HK is employed by a company that develops antibody therapeutics. JMR is employed by The Antibody Society, a non-profit trade association funded by corporate sponsors that develop antibody therapeutics or provide services to companies that develop antibody therapeutics, and she is Editor-in-Chief of
Figures
References
-
- Centers for Disease Control and Prevention . SARS-CoV-2Variant classifications and definitions. [Updated 2021. Oct 4 [accessed 2021 Nov 1]. www.cdc.gov/coronavirus/2019-ncov/variants/variant-info.html.
-
- U.S. Food and Drug Administration . Emergency use authorization. [Updated 2021. Oct 29 accessed 2021 Nov 1]. www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
