Antischistosomal tetrahydro-γ-carboline sulfonamides
- PMID: 35031451
- PMCID: PMC8826590
- DOI: 10.1016/j.bmcl.2022.128546
Antischistosomal tetrahydro-γ-carboline sulfonamides
Abstract
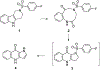
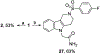
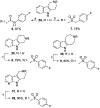
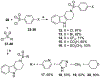
We discovered tetrahydro-γ-carboline sulfonamides as a new antischistosomal chemotype. The aryl sulfonamide and tetrahydro-γ-carboline substructures were required for high antischistosomal activity. Increasing polarity improved solubility and metabolic stability but decreased antischistosomal activity. We identified two compounds with IC50 values <5 µM against ex vivo Schistosoma mansoni.
Keywords: Antischistosomal; SAR; Sulfonamides; Tetrahydro-γ-carboline.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Figures
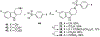
References
-
- Schistosomiasis Gryseels B.. Infect Dis Clin North Am. 2012;26:383–397. - PubMed
-
- Utzinger J, N’goran EK, Caffrey CR, Keiser J. From innovation to application: Social-ecological context, diagnostics, drugs and integrated control of schistosomiasis. Acta Trop. 2011;120:S121–S137. - PubMed
-
- Olliaro P, Delgado-Romero P, Keiser J. The little we know about the pharmacokinetics and pharmacodynamics of praziquantel (racemate and R-enantiomer). J Antimicrob Chemother. 2014;69:863–870. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

