Cabozantinib plus Nivolumab Phase I Expansion Study in Patients with Metastatic Urothelial Carcinoma Refractory to Immune Checkpoint Inhibitor Therapy
- PMID: 35031545
- PMCID: PMC9365339
- DOI: 10.1158/1078-0432.CCR-21-3726
Cabozantinib plus Nivolumab Phase I Expansion Study in Patients with Metastatic Urothelial Carcinoma Refractory to Immune Checkpoint Inhibitor Therapy
Abstract
Purpose: This study investigated the efficacy and tolerability of cabozantinib plus nivolumab (CaboNivo) in patients with metastatic urothelial carcinoma (mUC) that progressed on checkpoint inhibition (CPI).
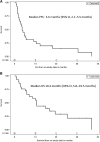
Patients and methods: A phase I expansion cohort of patients with mUC who received prior CPI was treated with cabozantinib 40 mg/day and nivolumab 3 mg/kg every 2 weeks until disease progression/unacceptable toxicity. The primary goal was objective response rate (ORR) per RECIST v.1.1. Secondary objectives included progression-free survival (PFS), duration of response (DoR), overall survival (OS), safety, and tolerability.
Results: Twenty-nine out of 30 patients enrolled were evaluable for efficacy. Median follow-up was 22.2 months. Most patients (86.7%) received prior chemotherapy and all patients received prior CPI (median seven cycles). ORR was 16.0%, with one complete response and three partial responses (PR). Among 4 responders, 2 were primary refractory, 1 had a PR, and 1 had stable disease on prior CPI. Median DoR was 33.5 months [95% confidence interval (CI), 3.7-33.5], median PFS was 3.6 months (95% CI, 2.1-5.5), and median OS was 10.4 months (95% CI, 5.8-19.5). CaboNivo decreased immunosuppressive subsets such as regulatory T cells (Tregs) and increased potential antitumor immune subsets such as nonclassical monocytes and effector T cells. A lower percentage of monocytic myeloid-derived suppressor cells (M-MDSC) and polymorphonuclear MDSCs, lower CTLA-4 and TIM-3 expression on Tregs, and higher effector CD4+ T cells at baseline were associated with better PFS and/or OS.
Conclusions: CaboNivo was clinically active, well tolerated, and favorably modulated peripheral blood immune subsets in patients with mUC refractory to CPI.
Trial registration: ClinicalTrials.gov NCT03866382 NCT02496208.
©2022 The Authors; Published by the American Association for Cancer Research.
Figures


References
-
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin 2021;71:7–33. - PubMed
-
- Nadal R, Bellmunt J. Management of metastatic bladder cancer. Cancer Treat Rev 2019;76:10–21. - PubMed
-
- Galsky MD, Arija JA, Bamias A, Davis ID, De Santis M, Kikuchi E, et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2020;395:1547–57. - PubMed
-
- Powles T, Park SH, Voog E, Caserta C, Valderrama BP, Gurney H, et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med 2020;383:1218–30. - PubMed
-
- Loriot Y, Necchi A, Park SH, Garcia-Donas J, Huddart R, Burgess E, et al. Erdafitinib in locally advanced or metastatic urothelial carcinoma. N Engl J Med 2019;381:338–48. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

