A Natural Polyphenol Exerts Antitumor Activity and Circumvents Anti-PD-1 Resistance through Effects on the Gut Microbiota
- PMID: 35031549
- PMCID: PMC9394387
- DOI: 10.1158/2159-8290.CD-21-0808
A Natural Polyphenol Exerts Antitumor Activity and Circumvents Anti-PD-1 Resistance through Effects on the Gut Microbiota
Abstract
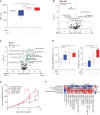
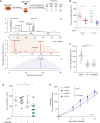
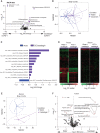
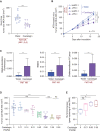
Several approaches to manipulate the gut microbiome for improving the activity of cancer immune-checkpoint inhibitors (ICI) are currently under evaluation. Here, we show that oral supplementation with the polyphenol-rich berry camu-camu (CC; Myrciaria dubia) in mice shifted gut microbial composition, which translated into antitumor activity and a stronger anti-PD-1 response. We identified castalagin, an ellagitannin, as the active compound in CC. Oral administration of castalagin enriched for bacteria associated with efficient immunotherapeutic responses (Ruminococcaceae and Alistipes) and improved the CD8+/FOXP3+CD4+ ratio within the tumor microenvironment. Moreover, castalagin induced metabolic changes, resulting in an increase in taurine-conjugated bile acids. Oral supplementation of castalagin following fecal microbiota transplantation from ICI-refractory patients into mice supported anti-PD-1 activity. Finally, we found that castalagin binds to Ruminococcus bromii and promoted an anticancer response. Altogether, our results identify castalagin as a polyphenol that acts as a prebiotic to circumvent anti-PD-1 resistance.
Significance: The polyphenol castalagin isolated from a berry has an antitumor effect through direct interactions with commensal bacteria, thus reprogramming the tumor microenvironment. In addition, in preclinical ICI-resistant models, castalagin reestablishes the efficacy of anti-PD-1. Together, these results provide a strong biological rationale to test castalagin as part of a clinical trial. This article is highlighted in the In This Issue feature, p. 873.
©2022 The Authors; Published by the American Association for Cancer Research.
Figures



Comment in
- Cancer Discov. 12:873.
- Cancer Discov. 12:873.
References
-
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis Fet al. . Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 2018;378:2078–92. - PubMed
-
- Pitt JM, Vétizou M, Daillère R, Roberti MP, Yamazaki T, Routy Bet al. . Resistance mechanisms to immune-checkpoint blockade in cancer: tumor-intrinsic and -extrinsic factors. Immunity 2016;44:1255–69. - PubMed
-
- Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature 2017;541:321–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

