Mining for novel cyclomaltodextrin glucanotransferases unravels the carbohydrate metabolism pathway via cyclodextrins in Thermoanaerobacterales
- PMID: 35031648
- PMCID: PMC8760340
- DOI: 10.1038/s41598-021-04569-x
Mining for novel cyclomaltodextrin glucanotransferases unravels the carbohydrate metabolism pathway via cyclodextrins in Thermoanaerobacterales
Abstract
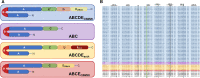
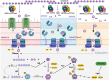
Carbohydrate metabolism via cyclodextrins (CM-CD) is an uncommon starch-converting pathway that thoroughly depends on extracellular cyclomaltodextrin glucanotransferases (CGTases) to transform the surrounding starch substrate to α-(1,4)-linked oligosaccharides and cyclodextrins (CDs). The CM-CD pathway has emerged as a convenient microbial adaptation to thrive under extreme temperatures, as CDs are functional amphipathic toroids with higher heat-resistant values than linear dextrins. Nevertheless, although the CM-CD pathway has been described in a few mesophilic bacteria and archaea, it remains obscure in extremely thermophilic prokaryotes (Topt ≥ 70 °C). Here, a new monophyletic group of CGTases with an exceptional three-domain ABC architecture was detected by (meta)genome mining of extremely thermophilic Thermoanaerobacterales living in a wide variety of hot starch-poor environments on Earth. Functional studies of a representative member, CldA, showed a maximum activity in a thermoacidophilic range (pH 4.0 and 80 °C) with remarkable product diversification that yielded a mixture of α:β:γ-CDs (34:62:4) from soluble starch, as well as G3-G7 linear dextrins and fermentable sugars as the primary products. Together, comparative genomics and predictive functional analysis, combined with data of the functionally characterized key proteins of the gene clusters encoding CGTases, revealed the CM-CD pathway in Thermoanaerobacterales and showed that it is involved in the synthesis, transportation, degradation, and metabolic assimilation of CDs.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Crini G. Review: A history of cyclodextrins. Chem. Rev. 2014;114:10940–10975. - PubMed
-
- Jambhekar SS, Breen P. Cyclodextrins in pharmaceutical formulations I: Structure and physicochemical properties, formation of complexes, and types of complex. Drug Discov. Today. 2016;21:356–362. - PubMed
-
- Strompen S, Miranda-Molina A, López-Munguía A, Castillo E, Saab-Rincón G. Acceptor-induced modification of regioselectivity in CGTase-catalyzed glycosylations of p-nitrophenyl-glucopyranosides. Carbohydr. Res. 2015;404:46–54. - PubMed
-
- Fan J, Zang Y, Jiang J, Lei J, Xue H. Beta-cyclodextrin-functionalized CdS nanorods as bulding modules for ultrasensitive photoelectrochemical bioassay of HIV DNA. Biosens. Bioelectron. 2019;142:1–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

