Transiently hypoxic tumour cell turnover and radiation sensitivity in human tumour xenografts
- PMID: 35031765
- PMCID: PMC9130130
- DOI: 10.1038/s41416-021-01691-5
Transiently hypoxic tumour cell turnover and radiation sensitivity in human tumour xenografts
Abstract
Background: Solid tumour perfusion can be unstable, creating transiently hypoxic cells that can contribute to radiation resistance. We investigated the in vivo lifetime of transiently hypoxic tumour cells and chronically hypoxic tumour cells during tumour growth and following irradiation.
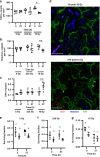
Methods: Hypoxic cells in SiHa and WiDr human tumour xenografts were labelled using pimonidazole and EF5, and turnover was quantified as the loss of labelled cells over time. The perfusion-modifying drug pentoxifylline was used to reoxygenate transiently hypoxic cells prior to hypoxia marker administration or irradiation.
Results: Chronically hypoxic cells constantly turnover in SiHa and WiDr tumours, with half-lives ranging from 42-82 h and significant numbers surviving >96 h. Transiently hypoxic cells constitute 26% of the total hypoxic cells in WiDr tumours. These transiently hypoxic cells survive at least 24 h, but then rapidly turnover with a half-life of 34 h and are undetectable 72 h after labelling. Transiently hypoxic cells are radiation-resistant, although vascular dysfunction induced by 10 Gy of ionising radiation preferentially kills transiently hypoxic cells.
Conclusions: Transiently hypoxic tumour cells survive up to 72 h in WiDr tumours and are radiation-resistant, although transiently hypoxic cells are sensitive to vascular dysfunction induced by high doses of ionising radiation.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Höckel M, Schlenger K, Aral B, Mitze M, Schäffer U, Vaupel P. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res. 1996;56:4509–15. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

