Alternative transcription and feedback regulation suggest that SlIDI1 is involved in tomato carotenoid synthesis in a complex way
- PMID: 35031800
- PMCID: PMC8788357
- DOI: 10.1093/hr/uhab045
Alternative transcription and feedback regulation suggest that SlIDI1 is involved in tomato carotenoid synthesis in a complex way
Abstract
Carotenoid pigments confer photoprotection and visual attraction and serve as precursors for many important signaling molecules. Herein, the orange-fruited phenotype of a tomato elite inbred line resulting from sharply reduced carotenoid levels and an increased β-carotene-to-lycopene ratio in fruit was shown to be controlled by a single recessive gene, oft3. BSA-Seq combined with fine mapping delimited the oft3 gene to a 71.23 kb interval on chromosome 4, including eight genes. Finally, the oft3 candidate gene SlIDI1, harboring a 116 bp deletion mutation, was identified by genome sequence analysis. Further functional complementation and CRISPR-Cas9 knockout experiments confirmed that SlIDI1 was the gene underlying the oft3 locus. qRT-PCR analysis revealed that the expression of SlIDI1 was highest in flowers and fruit and increased with fruit ripening or flower maturation. SlIDI1 simultaneously produced long and short transcripts by alternative transcription initiation and alternative splicing. Green fluorescent protein fusion expression revealed that the long isoform was mainly localized in plastids and that an N-terminal 59-amino acid extension sequence was responsible for plastid targeting. Short transcripts were identified in leaves and fruit by 5' RACE and in fruit by 3' RACE, which produced corresponding proteins lacking transit peptides and/or putative peroxisome targeting sequences, respectively. In SlIDI1 mutant fruit, SlBCH1 transcription involved in β-carotenoid catabolism was obviously suppressed, which may be responsible for the higher β-carotene-to-lycopene ratio and suggested potential feedback regulatory mechanisms involved in carotenoid pathway flux.
© The Author(s) 2022. Published by Oxford University Press on behalf of Nanjing Agricultural University.
Figures




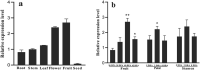



References
-
- Demmig-Adams B, Adams WW 3rd.. Antioxidants in photosynthesis and human nutrition. Science. 2002;298:2149–53. - PubMed
-
- Fray RG, Grierson D. Identification and genetic analysis of normal and mutant phytoene synthase genes of tomato by sequencing, complementation and co-suppression. Plant Mol Biol. 1993;22:589–602. - PubMed
LinkOut - more resources
Full Text Sources

