What do we know about dynamic glucose-enhanced (DGE) MRI and how close is it to the clinics? Horizon 2020 GLINT consortium report
- PMID: 35032288
- PMCID: PMC8901523
- DOI: 10.1007/s10334-021-00994-1
What do we know about dynamic glucose-enhanced (DGE) MRI and how close is it to the clinics? Horizon 2020 GLINT consortium report
Abstract
Cancer is one of the most devastating diseases that the world is currently facing, accounting for 10 million deaths in 2020 (WHO). In the last two decades, advanced medical imaging has played an ever more important role in the early detection of the disease, as it increases the chances of survival and the potential for full recovery. To date, dynamic glucose-enhanced (DGE) MRI using glucose-based chemical exchange saturation transfer (glucoCEST) has demonstrated the sensitivity to detect both D-glucose and glucose analogs, such as 3-oxy-methyl-D-glucose (3OMG) uptake in tumors. As one of the recent international efforts aiming at pushing the boundaries of translation of the DGE MRI technique into clinical practice, a multidisciplinary team of eight partners came together to form the "glucoCEST Imaging of Neoplastic Tumors (GLINT)" consortium, funded by the Horizon 2020 European Commission. This paper summarizes the progress made to date both by these groups and others in increasing our knowledge of the underlying mechanisms related to this technique as well as translating it into clinical practice.
Keywords: 3-Oxy-methyl-D-glucose; CEST; Cancer; DGE MRI; Glucose; MRI; glucoCEST.
© 2022. The Author(s).
Conflict of interest statement
Dr X. Golay is CEO, founder and shareholder of Gold Standard Phantoms.
Figures


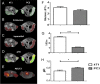
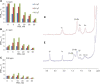
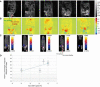
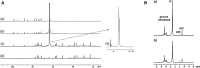
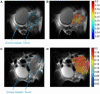

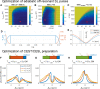



References
-
- Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. - PubMed
-
- Etzioni R, Urban N, Ramsey S, McIntosh M, Schwartz S, Reid B, Radich J, Anderson G, Hartwell L. The case for early detection. Nat Rev Cancer. 2003;3(4):243–252. - PubMed
-
- Day SE, Kettunen MI, Gallagher FA, Hu DE, Lerche M, Wolber J, Golman K, Ardenkjaer-Larsen JH, Brindle KM. Detecting tumor response to treatment using hyperpolarized 13C magnetic resonance imaging and spectroscopy. Nat Med. 2007;13(11):1382–1387. - PubMed
-
- Gambhir SS, Czernin J, Schwimmer J, Silverman DH, Coleman RE, Phelps ME. A tabulated summary of the FDG PET literature. J Nucl Med. 2001;42(5 Suppl):1S–93S. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

