Patient-reported outcomes with first-line nivolumab plus cabozantinib versus sunitinib in patients with advanced renal cell carcinoma treated in CheckMate 9ER: an open-label, randomised, phase 3 trial
- PMID: 35032437
- PMCID: PMC9479564
- DOI: 10.1016/S1470-2045(21)00693-8
Patient-reported outcomes with first-line nivolumab plus cabozantinib versus sunitinib in patients with advanced renal cell carcinoma treated in CheckMate 9ER: an open-label, randomised, phase 3 trial
Abstract
Background: In the CheckMate 9ER trial, patients with advanced renal cell carcinoma who received first-line nivolumab plus cabozantinib had significantly better progression-free survival compared with those given sunitinib. In this study, we aimed to describe the patient-reported outcome (PRO) results from CheckMate 9ER.
Methods: In this open-label, randomised, phase 3 trial done in 125 cancer centres, urology centres, and hospitals across 18 countries, patients aged 18 years or older with previously untreated advanced renal cell carcinoma with a clear-cell component, a Karnofsky performance status of 70% or more, and available tumour tissue were randomly assigned (1:1) via interactive response technology to nivolumab 240 mg intravenously every 2 weeks plus oral cabozantinib 40 mg per day, or oral sunitinib 50 mg per day monotherapy for 4 weeks in 6-week cycles. The primary endpoint of progression-free survival was reported previously. PROs were analysed as prespecified exploratory endpoints at common timepoints (at baseline and every 6 weeks) until week 115. Disease-related symptoms were evaluated using the 19-item Functional Assessment of Cancer Therapy-Kidney Symptom Index (FKSI-19), and global health status was assessed with the three-level EQ-5D (EQ-5D-3L) visual analogue scale (VAS) and UK utility index. PRO analyses were done in the intention-to-treat population. Change from baseline was assessed using mixed-model repeated measures. A time-to-deterioration analysis was done for first and confirmed deterioration events. This study is registered with ClinicalTrials.gov, NCT03141177, and is closed to recruitment.
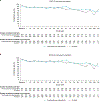
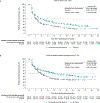
Findings: Between Sept 11, 2017, and May 14, 2019, 323 patients were randomly assigned to nivolumab plus cabozantinib and 328 to sunitinib. Median follow-up was 23·5 months (IQR 21·0-26·5). At baseline, patients in both groups reported low symptom burden (FKSI-19 disease-related symptoms version 1 mean scores at baseline were 30·24 [SD 5·19] for the nivolumab plus cabozantinib group and 30·06 [5·03] for the sunitinib group). Change from baseline in PRO scores indicated that nivolumab plus cabozantinib was associated with more favourable outcomes versus sunitinib (treatment difference 2·38 [95% CI 1·20-3·56], nominal p<0·0001, effect size 0·33 [95% CI 0·17-0·50] for FKSI-19 total score; 1·33 [0·84-1·83], nominal p<0·0001, 0·45 [0·28-0·61] for FKSI-19 disease-related symptoms version 1; 3·48 [1·58-5·39], nominal p=0·0004, 0·30 [0·14-0·47] for EQ-5D-3L VAS; and 0·04 [0·01-0·07], nominal p=0·0036, 0·25 [0·08-0·41] for EQ-5D-3L UK utility index), reaching significance at most timepoints. Nivolumab plus cabozantinib was associated with decreased risk of clinically meaningful deterioration for FKSI-19 total score compared with sunitinib (first deterioration event hazard ratio 0·70 [95% CI 0·56-0·86], nominal p=0·0007; confirmed deterioration event 0·63 [0·50-0·80], nominal p=0·0001).
Interpretation: PROs were maintained or improved with nivolumab plus cabozantinib versus sunitinib. Compared with sunitinib, nivolumab plus cabozantinib significantly delayed time to deterioration of patient-reported outcome scores. These results suggest a benefit for nivolumab plus cabozantinib compared with sunitinib in the treatment of patients with advanced renal cell carcinoma.
Funding: Bristol Myers Squibb.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests DC reports consultant fees from AbbVie, Bristol Myers Squibb (BMS), Exelixis, Merck, Novartis, and Pfizer; reports licensing fees from FACIT.org; research grants (institutional) from AbbVie, Astellas, Aveo, BMS, GlaxoSmithKline, Merck, Novartis, and Pfizer; and is an officer of FACIT.org. RJM reports advisory board fees from AstraZeneca, Aveo Pharmaceuticals, Eisai, EMD Serono, Exelixis, Genentech/Roche, Incyte, Lilly Oncology, Merck, Novartis, and Pfizer; and principal investigator fees (institutional) from BMS, Eisai, Exelixis, Genentech/Roche, Merck, and Pfizer. CS reports advisory board fees from Astellas Pharma, Bayer, BMS, EUSA Pharma, Hoffmann-La Roche, Ipsen, Merck Sharp & Dohme, Novartis, Pfizer, and Sanofi-Aventis; speakers' bureau fees from Astellas Pharma, BMS, Hoffmann-La Roche, Ipsen, and Pfizer; and research grants (institutional) from AB Science, Aragon Pharmaceuticals, Astellas Pharma, AstraZeneca, Bayer, Blueprint Medicines, Boehringer Ingelheim España, BMS, Clovis Oncology, Exelixis, Genentech, GlaxoSmithKline, Hoffmann-La Roche, Novartis Farmaceutica, Pfizer, and Sanofi-Aventis. SIB is an employee of BMS, and has stock ownership in BMS and GlaxoSmithKline. FE, MH, BS, and JZ are employees of and have stock ownership in BMS. JFW is an employee and has stock ownership in Exelixis. CI is an employee of IQVIA. TKC reports advisory board fees from Aptitude Health, AstraZeneca, BMS, Calithera, EMD Serono, Exelixis, Infinity, Merck, Pfizer, and Surface Oncology; speaker's fees from Advent Health, ASCO-SITC, ASiM, CancerNet, Ipsen, MD Anderson Cancer Center (Houston, TX, USA), MJH Life Sciences, PeerView, Physicians Education Resources, Research To Practice, Springer, and WebMD; consulting fees from The Analysis Group; stock ownership in Pionyr and Tempest; a personal grant from Orien; royalties from Up-To-Date online textbook; funding (institutional) from Alliance Cooperative Group, AstraZeneca, BMS, Eisai, EMD Serono, Exelixis, Lilly, Merck, Peloton, Pfizer, Takeda, and Tracon; research grants (institutional) from BMS, Exelixis, and Roche; principal investigator fees (institutional) from GlaxoSmithKline, Roche, and Surface Oncology; a non-financial leadership role interest with Kidney Cancer Research Summit of KidneyCAN (meeting co-chair in 2019 and 2020); being a grant reviewer for the American Association for Cancer Research; being a track leader, session chair, speaker, and discussant for the American Society of Clinical Oncology; being a speaker and discussant for the European Society for Medical Oncology; access to the genomic database from Foundation Medicine; access to the genomic database (institutional) from Guardant; access to the genomic database (institutional) from Invitae; personal non-financial interests for medical writing and editorial assistance from Clinical Thinking, Envision Pharma Group, Fishawack Group of Companies, Health Interactions, Parexel, and Oxford PharmaGenesis; and personal non-financial interests for reviews of papers for various journals. ABA declares no competing interests.
Figures




Comment in
-
Patient-reported outcomes: what really matters to patients?Lancet Oncol. 2022 May;23(5):e198. doi: 10.1016/S1470-2045(22)00156-5. Lancet Oncol. 2022. PMID: 35489342 No abstract available.
-
Patient-reported outcomes: what really matters to patients? - Authors' reply.Lancet Oncol. 2022 May;23(5):e199. doi: 10.1016/S1470-2045(22)00216-9. Lancet Oncol. 2022. PMID: 35489343 No abstract available.
References
-
- Kotecha RR, Motzer RJ, Voss MH. Towards individualized therapy for metastatic renal cell carcinoma. Nat Rev Clin Oncol 2019; 16: 621–33. - PubMed
-
- Choueiri TK, Motzer RJ. Systemic therapy for metastatic renal-cell carcinoma. N Engl J Med 2017; 376: 354–66. - PubMed
-
- Gurram S, Al Harthy M, Ball MW. The changing landscape of systemic therapy in metastatic renal cell carcinoma: an update. Discov Med 2020; 29: 191–9. - PubMed
-
- Bedke J, Albiges L, Capitanio U, et al. Updated European Association of Urology guidelines on renal cell carcinoma: Nivolumab plus cabozantinib joins immune checkpoint inhibition combination therapies for treatment-naïve metastatic clear-cell renal cell carcinoma. Eur Urol 2021; 79: 339–42. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

