Myofibril orientation as a metric for characterizing heart disease
- PMID: 35032456
- PMCID: PMC8874025
- DOI: 10.1016/j.bpj.2022.01.009
Myofibril orientation as a metric for characterizing heart disease
Abstract
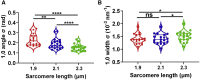
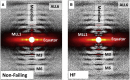
Myocyte disarray is a hallmark of many cardiac disorders. However, the relationship between alterations in the orientation of individual myofibrils and myofilaments to disease progression has been largely underexplored. This oversight has predominantly been because of a paucity of methods for objective and quantitative analysis. Here, we introduce a novel, less-biased approach to quantify myofibrillar and myofilament orientation in cardiac muscle under near-physiological conditions and demonstrate its superiority as compared with conventional histological assessments. Using small-angle x-ray diffraction, we first investigated changes in myofibrillar orientation at increasing sarcomere lengths in permeabilized, relaxed, wild-type mouse myocardium from the left ventricle by assessing the angular spread of the 1,0 equatorial reflection (angle σ). At a sarcomere length of 1.9 μm, the angle σ was 0.23 ± 0.01 rad, decreased to 0.19 ± 0.01 rad at a sarcomere length of 2.1 μm, and further decreased to 0.15 ± 0.01 rad at a sarcomere length of 2.3 μm (p < 0.0001). Angle σ was significantly larger in R403Q, a MYH7 hypertrophic cardiomyopathy model, porcine myocardium (0.24 ± 0.01 rad) compared with wild-type myocardium (0.14 ± 0.005 rad; p < 0.0001), as well as in human heart failure tissue (0.19 ± 0.006 rad) when compared with nonfailing samples (0.17 ± 0.007 rad; p = 0.01). These data indicate that diseased myocardium suffers from greater myofibrillar disorientation compared with healthy controls. Finally, we showed that conventional, histology-based analysis of disarray can be subject to user bias and/or sampling error and lead to false positives. Our method for directly assessing myofibrillar orientation avoids the artifacts introduced by conventional histological approaches that assess myocyte orientation and only indirectly evaluate myofibrillar orientation, and provides a precise and objective metric for phenotypically characterizing myocardium. The ability to obtain excellent x-ray diffraction patterns from frozen human myocardium provides a new tool for investigating structural anomalies associated with cardiac diseases.
Copyright © 2022 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

