How I treat biliary tract cancer
- PMID: 35032765
- PMCID: PMC8762076
- DOI: 10.1016/j.esmoop.2021.100378
How I treat biliary tract cancer
Abstract
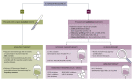
Management of biliary tract cancers (BTCs) is rapidly evolving. Curative management relies on surgical resection followed by adjuvant capecitabine for cholangiocarcinoma and gallbladder cancers. Unfortunately relapse rate remains high, and better adjuvant strategies are urgently required. A majority of patients are diagnosed with advanced disease, when chemotherapy with cisplatin and gemcitabine followed by second-line 5-FU and oxaliplatin /irinotecan is the cornerstone of treatment for most patients in the absence of targetable alterations. Targeted therapies, including therapies for tumours with fibroblast growth factor receptor-2 (FGFR-2) fusions, isocitrate dehydrogenase-1 (IDH-1) mutations, B-Raf proto-oncogene serine/threonine kinase (BRAF) V600E mutations, neurotrophic tyrosine receptor kinase (NTRK) fusions, Human epidermal growth factor-2 (HER-2) amplifications, and/or microsatellite instability are rapidly changing the treatment paradigm for many patients with advanced BTC, especially for patients with intrahepatic cholangiocarcinoma. Because of this, molecular profiling should be considered early on patients pathway to allow adequate planning of therapy. Ongoing research is likely to clarify the role of immunotherapy, liver-directed therapy, and liver transplant for BTCs in the future.
Keywords: ampullary cancer; biliary tract cancer; chemotherapy; cholangiocarcinoma; gallbladder cancer; surgery; targeted; treatment.
Copyright © 2021 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Disclosure AL has received travel and educational support from Ipsen, Pfizer, Bayer, AAA, Sirtex, Novartis, Mylan, and Delcath; speaker honoraria from Merck, Pfizer, Ipsen, Incyte, AAA, QED, Servier, and EISAI; advisory honoraria from EISAI, Nutricia Ipsen, QED, Roche, Servier, and Boston Scientific; is a member of the Knowledge Network and NETConnect Initiatives funded by Ipsen. JE has received honoraria from Servier, Incyte, Basilea, Boston Scientific, MSD, AstraZeneca, Roche, Eisai, Ipsen, and Bayer. LG reports research funding (to the institution) from Adaptimmune, Bayer, Merck, MacroGenics, Genentech, Novartis, Incyte, Loxo Oncology, Relay Therapeutics, QED, Taiho Oncology, Leap Therapeutics, Bristol Myers Squibb, NuCana, and Servier; honoraria (to self) for serving on scientific advisory committees or as a consultant from Alentis Therapeutics AG, Black Diamond, Basilea, Genentech, Exelixis, H3Biomedicine, Incyte Corporation, QED Therapeutics, Sirtex Medical Ltd, The Servier Group, Sirtex, and Taiho Oncology and participation on data safety monitoring boards for AstraZeneca.
Figures

References
-
- Valle J.W., Borbath I., Khan S.A., et al. Biliary cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(suppl 5):v28–v37. - PubMed
-
- Forner A., Vidili G., Rengo M., Bujanda L., Ponz-Sarvisé M., Lamarca A. Clinical presentation, diagnosis and staging of cholangiocarcinoma. Liver Int. 2019;39(Suppl 1):98–107. - PubMed
-
- Lamarca A., Barriuso J., Chander A., et al. 18F-fluorodeoxyglucose positron emission tomography (18FDG-PET) for patients with biliary tract cancer: systematic review and meta-analysis. J Hepatol. 2019;71(1):115–129. - PubMed
-
- Primrose J.N., Fox R.P., Palmer D.H., et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019;20(5):663–673. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

