Impaired seroconversion after SARS-CoV-2 mRNA vaccines in patients with solid tumours receiving anticancer treatment
- PMID: 35032813
- PMCID: PMC8692068
- DOI: 10.1016/j.ejca.2021.12.006
Impaired seroconversion after SARS-CoV-2 mRNA vaccines in patients with solid tumours receiving anticancer treatment
Abstract
Background: Patients with solid tumours have high COVID-19 mortality. Limited and heterogeneous data are available regarding the immunogenicity of SARS-CoV-2 mRNA vaccines in this population.
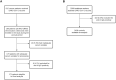
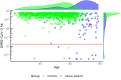
Methods and findings: This is a prospective, single-centre cohort study aiming at evaluating seroconversion in terms of anti-spike antibodies in a population of patients with solid tumours undergoing cancer therapy within 2 months before the second vaccine dose, as compared with a cohort of controls. Subjects who were not SARS-CoV-2 naïve were excluded, and 171 patients were included in the final study population (150 vaccinated with BNT162b2, 87.7%; 21 with mRNA-1273, 12.3%) and compared with 2406 controls. The median follow-up time from the second dose of vaccination was 30 days (12-42; IQR: 26-34). Most patients had metastatic disease (138, 80.7%). Seroconversion rate was significantly lower in cancer patients than in controls (94.2% versus 99.8%, p < 0.001). At univariate logistic regression analysis, Odds ratio (OR) for seroconversion was also reduced in older individuals (>70 years). A multivariate logistic model confirmed cancer as the only significant variable in impairing seroconversion (OR 0.03, p < 0.001). In the cancer population, a multivariate analysis among clinical variables, including the type of cancer treatment, showed ECOG PS > 2 as the only one of impact (OR 0.07, p = 0.012).
Conclusions: There is a fraction of 6% of patients with solid tumours undergoing cancer treatment, mainly with poorer performance status, who fail to obtain seroconversion after SARS-CoV-2 mRNA vaccines. These patients should be considered for enhanced vaccination strategies and carefully monitored for SARS-CoV-2 infection during cancer treatment.
Keywords: COVID-19; Cancer; Chemotherapy; Immunogenicity; Immunotherapy; Seroconversion; Vaccine.
Copyright © 2021 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Conflict of interest statement The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
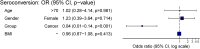
Comment in
-
Seroconversion after SARS-CoV-2 mRNA booster vaccine in cancer patients.Eur J Cancer. 2022 May;167:175-176. doi: 10.1016/j.ejca.2022.02.032. Epub 2022 Mar 15. Eur J Cancer. 2022. PMID: 35370047 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

