Determinants of early antibody responses to COVID-19 mRNA vaccines in a cohort of exposed and naïve healthcare workers
- PMID: 35032961
- PMCID: PMC8752368
- DOI: 10.1016/j.ebiom.2021.103805
Determinants of early antibody responses to COVID-19 mRNA vaccines in a cohort of exposed and naïve healthcare workers
Abstract
Background: Two doses of mRNA vaccination have shown >94% efficacy at preventing COVID-19 mostly in naïve adults, but it is not clear if the second dose is needed to maximize effectiveness in those previously exposed to SARS-CoV-2 and what other factors affect responsiveness.
Methods: We measured IgA, IgG and IgM levels against SARS-CoV-2 spike (S) and nucleocapsid (N) antigens from the wild-type and S from the Alpha, Beta and Gamma variants of concern, after BNT162b2 (Pfizer/BioNTech) or mRNA-1273 (Moderna) vaccination in a cohort of health care workers (N=578). Neutralizing capacity and antibody avidity were evaluated. Data were analyzed in relation to COVID-19 history, comorbidities, vaccine doses, brand and adverse events.
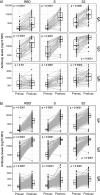
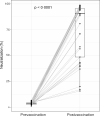
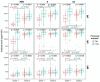
Findings: Vaccination induced robust IgA and IgG levels against all S antigens. Neutralization capacity and S IgA and IgG levels were higher in mRNA-1273 vaccinees, previously SARS-CoV-2 exposed, particularly if symptomatic, and in those experiencing systemic adverse effects (p<0·05). A second dose in pre-exposed did not increase antibody levels. Smoking and comorbidities were associated with 43% (95% CI, 19-59) and 45% (95% CI, 63-18) lower neutralization, respectively, and 35% (95% CI, 3-57%) and 55% (95% CI, 33-70%) lower antibody levels, respectively. Among fully vaccinated, 6·3% breakthroughs were detected up to 189 days post-vaccination. Among pre-exposed non-vaccinated, 90% were IgG seropositive more than 300 days post-infection.
Interpretation: Our data support administering a single-dose in pre-exposed healthy individuals as primary vaccination. However, heterogeneity of responses suggests that personalized recommendations may be necessary depending on COVID-19 history and life-style. Higher mRNA-1273 immunogenicity would be beneficial for those expected to respond worse to vaccination and in face of variants that escape immunity such as Omicron. Persistence of antibody levels in pre-exposed unvaccinated indicates maintenance of immunity up to one year.
Funding: This work was supported by Institut de Salut Global de Barcelona (ISGlobal) internal funds, in-kind contributions from Hospital Clínic de Barcelona, the Fundació Privada Daniel Bravo Andreu, and European Institute of Innovation and Technology (EIT) Health (grant number 20877), supported by the European Institute of Innovation and Technology, a body of the European Union receiving support from the H2020 Research and Innovation Programme. We acknowledge support from the Spanish Ministry of Science and Innovation and State Research Agency through the "Centro de Excelencia Severo Ochoa 2019-2023" Program (CEX2018-000806-S), and support from the Generalitat de Catalunya through the CERCA Program. L. I. work was supported by PID2019-110810RB-I00 grant from the Spanish Ministry of Science & Innovation. Development of SARS-CoV-2 reagents was partially supported by the National Institute of Allergy and Infectious Diseases Centers of Excellence for Influenza Research and Surveillance (contract number HHSN272201400008C). The funders had no role in study design, data collection and analysis, the decision to publish, or the preparation of the manuscript.
Keywords: Antibody; Avidity; COVID-19; Health care workers; Neutralization; SARS-CoV-2; mRNA vaccines.
Copyright © 2022 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


Similar articles
-
Follow-Up and Comparative Assessment of SARS-CoV-2 IgA, IgG, Neutralizing, and Total Antibody Responses After BNT162b2 or mRNA-1273 Heterologous Booster Vaccination.Influenza Other Respir Viruses. 2024 May;18(5):e13290. doi: 10.1111/irv.13290. Influenza Other Respir Viruses. 2024. PMID: 38706402 Free PMC article.
-
Anti-SARS-CoV-2 Immunoglobulin Isotypes, and Neutralization Activity Against Viral Variants, According to BNT162b2-Vaccination and Infection History.Front Immunol. 2021 Dec 17;12:793191. doi: 10.3389/fimmu.2021.793191. eCollection 2021. Front Immunol. 2021. PMID: 34975897 Free PMC article.
-
Antibody Response to SARS-CoV-2 Infection and Vaccination in COVID-19-naïve and Experienced Individuals.Viruses. 2022 Feb 10;14(2):370. doi: 10.3390/v14020370. Viruses. 2022. PMID: 35215962 Free PMC article.
-
mRNA Covid-19 vaccines in pregnancy: A systematic review.PLoS One. 2022 Feb 2;17(2):e0261350. doi: 10.1371/journal.pone.0261350. eCollection 2022. PLoS One. 2022. PMID: 35108277 Free PMC article.
-
"COVID-19/SARS-CoV-2 virus spike protein-related delayed inflammatory reaction to hyaluronic acid dermal fillers: a challenging clinical conundrum in diagnosis and treatment".Arch Dermatol Res. 2022 Jan;314(1):1-15. doi: 10.1007/s00403-021-02190-6. Epub 2021 Feb 9. Arch Dermatol Res. 2022. PMID: 33559733 Free PMC article. Review.
Cited by
-
Comparative Effectiveness of Bivalent (Original/Omicron BA.4/BA.5) COVID-19 Vaccines in Adults.Vaccines (Basel). 2023 Nov 11;11(11):1711. doi: 10.3390/vaccines11111711. Vaccines (Basel). 2023. PMID: 38006043 Free PMC article.
-
Down-regulation of SARS-CoV-2 neutralizing antibodies in vaccinated smokers.MedComm (2020). 2022 Aug 12;3(3):e166. doi: 10.1002/mco2.166. eCollection 2022 Sep. MedComm (2020). 2022. PMID: 35978853 Free PMC article. No abstract available.
-
Evaluation of the Diagnostic Performance of Two Automated SARS-CoV-2 Neutralization Immunoassays following Two Doses of mRNA, Adenoviral Vector, and Inactivated Whole-Virus Vaccinations in COVID-19 Naïve Subjects.Microorganisms. 2023 Apr 30;11(5):1187. doi: 10.3390/microorganisms11051187. Microorganisms. 2023. PMID: 37317161 Free PMC article.
-
The subdued post-boost spike-directed secondary IgG antibody response in Ugandan recipients of the Pfizer-BioNTech BNT162b2 vaccine has implications for local vaccination policies.Front Immunol. 2024 Feb 16;15:1325387. doi: 10.3389/fimmu.2024.1325387. eCollection 2024. Front Immunol. 2024. PMID: 38469296 Free PMC article.
-
Sustained seropositivity up to 20.5 months after COVID-19.BMC Med. 2022 Oct 13;20(1):379. doi: 10.1186/s12916-022-02570-3. BMC Med. 2022. PMID: 36224590 Free PMC article.
References
-
- WHO. COVID-19 vaccine tracker and landscape n.d. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-cand.... 2021
-
- Krammer F, Srivastava K, Team P, et al. Robust spike antibody responses and increased reactogenicity in seropositive individuals after a 1 single dose of SARS-CoV-2 mRNA vaccine 2 3. MedRxiv. 2021 2021.01.29.21250653.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

