K63 ubiquitination in immune signaling
- PMID: 35033428
- PMCID: PMC8755460
- DOI: 10.1016/j.it.2021.12.005
K63 ubiquitination in immune signaling
Abstract
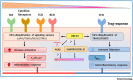
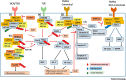
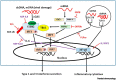
Ubc13-catalyzed K63 ubiquitination is a major control point for immune signaling. Recent evidence has shown that the control of multiple immune functions, including chronic inflammation, pathogen responses, lymphocyte activation, and regulatory signaling, is altered by K63 ubiquitination. In this review, we detail the novel cellular sensors that are dependent on K63 ubiquitination for their function in the immune signaling network. Many pathogens, including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), can target K63 ubiquitination to inhibit pathogen immune responses; we describe novel details of the pathways involved and summarize recent clinically relevant SARS-CoV-2-specific responses. We also discuss recent evidence that regulatory T cell (Treg) versus T helper (TH) 1 and TH17 cell subset regulation might involve K63 ubiquitination. Knowledge gaps that merit future investigation and clinically relevant pathways are also addressed.
Keywords: E3 ligase; K63-linked ubiquitination; SARS-CoV-2; Ubc13; immune response; immune tolerance; pattern recognition receptor (PRR); ubiquitin.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests J. Reed is an employee of Sanofi. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

