Comparative Bioavailability Study of a New Vitamin D3 Orodispersible Film Versus a Marketed Oral Solution in Healthy Volunteers
- PMID: 35034345
- PMCID: PMC8761109
- DOI: 10.1007/s40261-021-01113-7
Comparative Bioavailability Study of a New Vitamin D3 Orodispersible Film Versus a Marketed Oral Solution in Healthy Volunteers
Abstract
Background and objective: An orally disintegrating film (ODF) formulation of vitamin D3 that dissolves rapidly in the mouth without drinking or chewing may be a worthwhile alternative to currently available drug products for therapeutic vitamin D supplementation. This study aimed to compare the bioavailability of a single dose of a vitamin D3 25000 I.U. ODF with those of a marketed oral vitamin D3 preparation in healthy subjects.
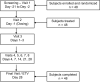
Methods: This Phase 1, randomised, parallel-group, open-label study compared the pharmacokinetics of calcifediol [25(OH)D3], the precursor of bioactive vitamin D3, after a single dose of a new vitamin D3 25,000 I.U. ODF with those of a Reference formulation (vitamin D3 25000 I.U./2.5 mL oral solution) in healthy adult subjects using a validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay. The primary objective was bioavailability under fed conditions, defined as maximum plasma concentration (Cmax) of 25(OH)D3 and area under the concentration-time curve from time zero to time t, the last quantifiable concentration (AUC0-t). The pharmacokinetics of 25(OH)D3 were also evaluated following the ODF administration under fasting conditions. Subjects were randomised to receive a single dose of the vitamin D3 25000 I.U. ODF or the Reference oral solution under fed conditions or the vitamin D3 ODF under fasting conditions.
Results: Forty-eight healthy subjects were randomised and completed the study. Overall, the pharmacokinetic profile was very similar across the three treatment groups, and bioavailability did not significantly differ among treatments. Under fed conditions, mean 25(OH)D3 plasma values for Cmax were 6.68 ± 2.03 versus 6.61 ± 2.62 ng/mL for the Test versus Reference formulations. Corresponding values for AUC0-t were 2364.80 ± 1336.97 versus 2150.52 ± 1622.76 ng/mL × h. Mean Cmax was slightly lower (6.68 ± 2.03 vs 7.23 ± 1.48 ng/mL) and the time to reach peak concentration was delayed (144 h [36-312] versus 42 h (2-480]) with the ODF under fed versus fasting conditions (p = 0.0371). The point estimates and 90 % CIs of the Testfed/Referencefed ratios of the geometric means showed that the bioavailability of exogenous 25(OH)D3 was, both in rate and extent of absorption, slightly higher with the vitamin D3 ODF than the vitamin D3 oral solution under the administration conditions recommended for the vitamin D3 oral solution. Palatability and ease of use of the ODF were satisfactory.
Conclusion: The new ODF 25000 I.U. formulation provided a valuable alternative to the marketed oral solution for therapeutic vitamin D supplementation, with a bioavailability that was slightly higher than that of the vitamin D3 oral solution administered under the same conditions.
Trial registration: The study was retrospectively registered with the ISRCTN Registry (Registry code: ISRCTN13208948) on 27 November 2020.
© 2022. The Author(s).
Conflict of interest statement
S.R., C.C, G.M are employees of IBSA Institut Biochimique S.A., Switzerland. I.C, F.M, A.M.G are employees of IBSA Farmaceutici Italia, Italy. M.R is an employee from CROSS Research S.A., Switzerland. The authors report no other conflicts of interest in this work.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources

