Safety and immunogenicity of a third-dose homologous BBIBP-CorV boosting vaccination: interim results from a prospective open-label study
- PMID: 35034582
- PMCID: PMC8881062
- DOI: 10.1080/22221751.2022.2025746
Safety and immunogenicity of a third-dose homologous BBIBP-CorV boosting vaccination: interim results from a prospective open-label study
Abstract
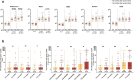
A COVID-19 booster vaccination is being comprehensively evaluated globally due to the emerging concern of reduced protection rate of previous vaccination and circulating Variants of Concern (VOC). But the safety and immunogenicity of homologous BBIBP-CorV boosting vaccination are yet to be thoroughly evaluated. We conducted this prospective, open-label study in Huashan Hospital using a third 6.5U BBIBP-CorV administered at an interval of 4-8 months following the previous two doses in healthy adults. Safety, anti-RBD response and neutralizing titers against SARS-CoV-2 and VOCs were examined. Sixty-three and forty participants entered the booster and the control group, respectively. A significant increase in IFN-γ SFU per million PBMCs was observed on day 14 against N peptide (20 vs. 5, P < 0.001). On day 14, pVNT GMTs increased over 15 folds of the baseline levels against prototype to reach 404.54 titers and over 9-13 folds against 4 VOCs and continuously increased by day 28. sVNT GMTs increased 112.51 and 127.45 folds by days 14 and 28 compared to the baseline level. Median anti-RBD antibody and IgG level significantly increased from 11.12 to 2607.50 BAU/ml and 4.07 to 619.20 BAU/ml on day 14. On day 14, females showed a significantly higher cell-mediated immune response against S1 peptide. The 7-8 months interval group had a higher humoral response than the 4-6 months interval group. No severe adverse event was reported. A third homologous BBIBP-CorV boosting vaccination was safe and highly immunogenic for healthy adults and broadened participants' immunity against VOCs.
Keywords: SARS-CoV-2 inactivated vaccines; booster vaccination shot; homologous; immunogenicity; safety.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures


References
-
- See how vaccinations are going in your County and State 2021 [updated October 28]. Available from: https://www.nytimes.com/interactive/2020/us/covid-19-vaccine-doses.html.
-
- Zeng G, Wu Q, Pan H, et al. Immunogenicity and CoronaVac safety of a third dose of, and schedule immune persistence of a two-dose, healthy in adults: single-centre interim results from two, double-blind, randomised, phase placebo-controlled 2 Dis clinical trials. Lancet Infect. 2021. Dec 7;S1473-3099(21)00681-2. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
