Reduced sensitivity of SARS-CoV-2 Omicron variant to antibody neutralization elicited by booster vaccination
- PMID: 35034952
- PMCID: PMC8761745
- DOI: 10.1038/s41421-022-00375-5
Reduced sensitivity of SARS-CoV-2 Omicron variant to antibody neutralization elicited by booster vaccination
Conflict of interest statement
The authors declare no competing interests.
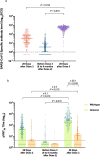
Figures
References
-
- Zeng, G. et al. Immunogenicity and safety of a third dose of CoronaVac, and immune persistence of a two-dose schedule, in healthy adults: interim results from two single-centre, double-blind, randomised, placebo-controlled phase 2 clinical trials. Lancet Infect. Dis.10.1016/S1473-3099(21)00681-2 (2021). - PMC - PubMed
-
- Pulliam, J. R. C. et al. Increased risk of SARS-CoV-2 reinfection associated with emergence of the Omicron variant in South Africa. medRxiv, 10.1101/2021.11.11.21266068 (2021).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

