Antibacterial and Antioxidant Effects of Magnesium Alloy on Titanium Dental Implants
- PMID: 35035523
- PMCID: PMC8758302
- DOI: 10.1155/2022/6537676
Antibacterial and Antioxidant Effects of Magnesium Alloy on Titanium Dental Implants
Retraction in
-
Retracted: Antibacterial and Antioxidant Effects of Magnesium Alloy on Titanium Dental Implants.Comput Math Methods Med. 2023 Dec 6;2023:9760718. doi: 10.1155/2023/9760718. eCollection 2023. Comput Math Methods Med. 2023. PMID: 38094419 Free PMC article.
Abstract
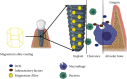
Objectives: In this study, a new type of dental implant by covering the surface of the titanium (Ti) implant with zinc-magnesium (Zn-Mg) alloy was designed, to study the antibacterial and antioxidant effects of Mg alloy on titanium (Ti) implants in oral implant restoration.
Methods: Human gingival fibroblasts (HGFs), S. sanguinis, and F. nucleatum bacteria were used to detect the bioactivity and antibacterial properties of Mg alloy-coated Ti implants. In addition, B6/J mice implanted with different materials were used to further detect their antibacterial and antioxidant properties.
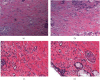
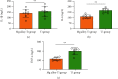
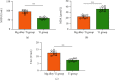
Results: The results showed that Mg alloy could better promote the adhesion and proliferation and improve the alkaline phosphatase (ALP) activity of HGFs, which contributed to better improved stability of implant osseointegration. In addition, Mg alloy could better inhibit the proliferation of S. sanguinis, while no significant difference was found in the proliferation of F. nucleatum between the two implants. In the mouse model, the peripheral inflammatory reaction and oxidative stress of the Mg alloy implant were significantly lower than those of the Ti alloy implant.
Conclusions: Zn-Mg alloy-coated Ti implants could better inhibit the growth of Gram-positive bacteria in the oral cavity, inhibit oxidative stress, and facilitate the proliferation activity of HGFs and the potential of osteoblast differentiation, thus, better increasing the stability of implant osseointegration.
Copyright © 2022 Yang Bai et al.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Multifunctions of dual Zn/Mg ion co-implanted titanium on osteogenesis, angiogenesis and bacteria inhibition for dental implants.Acta Biomater. 2017 Feb;49:590-603. doi: 10.1016/j.actbio.2016.11.067. Epub 2016 Nov 30. Acta Biomater. 2017. PMID: 27915020
-
Study on the Antibacterial Activity and Bone Inductivity of Nanosilver/PLGA-Coated TI-CU Implants.Int J Nanomedicine. 2024 Jun 24;19:6427-6447. doi: 10.2147/IJN.S456906. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38952675 Free PMC article.
-
Osseointegration of chitosan coated porous titanium alloy implant by reactive oxygen species-mediated activation of the PI3K/AKT pathway under diabetic conditions.Biomaterials. 2015 Jan;36:44-54. doi: 10.1016/j.biomaterials.2014.09.012. Epub 2014 Oct 11. Biomaterials. 2015. PMID: 25308520
-
Overview of Nanoparticle Coating of Dental Implants for Enhanced Osseointegration and Antimicrobial Purposes.J Pharm Pharm Sci. 2017;20(0):148-160. doi: 10.18433/J3GP6G. J Pharm Pharm Sci. 2017. PMID: 28554344 Review.
-
Does the incorporation of zinc into TiO2 on titanium surfaces increase bactericidal activity? A systematic review and meta-analysis.J Prosthet Dent. 2024 Sep;132(3):510-519. doi: 10.1016/j.prosdent.2022.05.007. Epub 2022 Oct 19. J Prosthet Dent. 2024. PMID: 36270807
Cited by
-
Enhancement of titanium surfaces using different acid solutions at room temperature to improve bone cell responses.J Dent Sci. 2025 Jan;20(1):373-383. doi: 10.1016/j.jds.2024.06.011. Epub 2024 Jun 26. J Dent Sci. 2025. PMID: 39873087 Free PMC article.
-
Retracted: Antibacterial and Antioxidant Effects of Magnesium Alloy on Titanium Dental Implants.Comput Math Methods Med. 2023 Dec 6;2023:9760718. doi: 10.1155/2023/9760718. eCollection 2023. Comput Math Methods Med. 2023. PMID: 38094419 Free PMC article.
-
Evaluation of Antibacterial, Antioxidant, Anti-inflammatory and Anticancer Efficacy of Titanium-Doped Graphene Oxide Nanoparticles.Cureus. 2024 Jan 6;16(1):e51737. doi: 10.7759/cureus.51737. eCollection 2024 Jan. Cureus. 2024. PMID: 38318546 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

