Pharmacokinetic and pharmacodynamic properties of ciprofol emulsion in Chinese subjects: a single center, open-label, single-arm dose-escalation phase 1 study
- PMID: 35035718
- PMCID: PMC8748126
Pharmacokinetic and pharmacodynamic properties of ciprofol emulsion in Chinese subjects: a single center, open-label, single-arm dose-escalation phase 1 study
Abstract
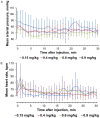
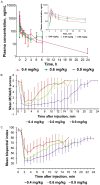
We conducted a single-center, single-arm, open-label, dose-escalation phase 1 clinical trial to evaluate the tolerability of a single intravenous injection of ciprofol emulsion for the induction of short-term general anesthesia. Four doses of ciprofol (0.15 mg/kg, n = 2; 0.4 mg/kg, n = 10; 0.6 mg/kg, n = 6; 0.9 mg/kg, n = 6) were administered. Twenty-four subjects were enrolled, with 18 subjects in the 0.4 to 0.9 mg/kg dosage groups included in the data analysis. In total, 37 mild and 4 moderate adverse events (AEs), including 9 abnormal limb movements (3 moderate cases), 8 cases of sinus bradycardia, 11 cases of prolonged QTcF interval (including 1 moderate case), and 1 case of hypotension, were found, but no serious AEs were reported. The Modified Observer's Assessment of Alertness/Sedation (MOAA/S) scores rapidly decreased after ciprofol administration. The duration of recovery of the verbal response, loss of verbal response duration, the duration of MOAA/S ≤1 and the duration until the return of responsiveness were all increased in a dose-dependent manner. The durations of bispectral index values <60 (6, 8 and 12 min) were similar to the durations of loss of verbal response (6, 8 and 14 min) and MOAA/S ≤1 (5, 5.5 and 13.5 min) in the 0.4, 0.6 and 0.9 mg/kg dose groups, respectively. The plasma concentration reached a peak value approximately 2 min after injection in the 0.4-0.9 mg/kg groups and all subjects fully recovered after ciprofol administration, with the shortest time being 9.2 min in the 0.4 mg/kg group. A ciprofol dosing regimen of 0.4-0.9 mg/kg was well-tolerated and exhibited rapid onset and recovery properties.
Keywords: Anesthetic; ciprofol; pharmacodynamics; pharmacokinetics; sedation.
AJTR Copyright © 2021.
Conflict of interest statement
Xiao Liu and Yong Liang were employees of Sichuan Haisco Pharmaceutical Group Co., Ltd. The remaining authors declared no conflicts of interest.
Figures
References
-
- White PF. Clinical uses of intravenous anesthetic and analgesic infusions. Anesth Analg. 1989;68:161–171. - PubMed
-
- Cockshott ID. Propofol (‘diprivan’) pharmacokinetics and metabolism--an overview. Postgrad Med J. 1985;61(Suppl 3):45–50. - PubMed
-
- Smith I, White PF, Nathanson M, Gouldson R. Propofol. An update on its clinical use. Anesthesiology. 1994;81:1005–1043. - PubMed
-
- Mahmoud M, Mason KP. Recent advances in intravenous anesthesia and anesthetics. F1000Res. 2018;7:F1000 Faculty Rev-470.
-
- Qin L, Ren L, Wan S, Liu G, Luo X, Liu Z, Li F, Yu Y, Liu J, Wei Y. Design, synthesis, and evaluation of novel 2,6-disubstituted phenol derivatives as general anesthetics. J Med Chem. 2017;60:3606–3617. - PubMed
LinkOut - more resources
Full Text Sources
