An introduction to inverse probability of treatment weighting in observational research
- PMID: 35035932
- PMCID: PMC8757413
- DOI: 10.1093/ckj/sfab158
An introduction to inverse probability of treatment weighting in observational research
Abstract
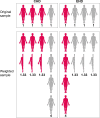
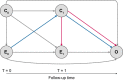
In this article we introduce the concept of inverse probability of treatment weighting (IPTW) and describe how this method can be applied to adjust for measured confounding in observational research, illustrated by a clinical example from nephrology. IPTW involves two main steps. First, the probability-or propensity-of being exposed to the risk factor or intervention of interest is calculated, given an individual's characteristics (i.e. propensity score). Second, weights are calculated as the inverse of the propensity score. The application of these weights to the study population creates a pseudopopulation in which confounders are equally distributed across exposed and unexposed groups. We also elaborate on how weighting can be applied in longitudinal studies to deal with informative censoring and time-dependent confounding in the setting of treatment-confounder feedback.
Keywords: chronic renal insufficiency; dialysis; epidemiology; guidelines; systematic review.
© The Author(s) 2021. Published by Oxford University Press on behalf of ERA.
Figures

References
-
- Stel VS, Jager KJ, Zoccali C et al. The randomized clinical trial: an unbeatable standard in clinical research? Kidney Int 2007; 72: 539–542 - PubMed
-
- Jager KJ, Stel VS, Wanner C et al. The valuable contribution of observational studies to nephrology. Kidney Int 2007; 72: 671–675 - PubMed
-
- Jager K, Zoccali C, MacLeod A et al. . Confounding: what it is and how to deal with it. Kidney Int 2008; 73: 256–260 - PubMed
-
- Tripepi G, Jager KJ, Dekker FW et al. Linear and logistic regression analysis. Kidney Int 2008; 73: 806–810 - PubMed
-
- Tripepi G, Jager KJ, Dekker FW et al. Stratification for confounding – part 1: the Mantel–Haenszel formula. Nephron Clin Pract 2010; 116: c317–c321 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

