Correlation between Reactogenicity and Immunogenicity after the ChAdOx1 nCoV-19 and BNT162b2 mRNA Vaccination
- PMID: 35036028
- PMCID: PMC8733188
- DOI: 10.4110/in.2021.21.e41
Correlation between Reactogenicity and Immunogenicity after the ChAdOx1 nCoV-19 and BNT162b2 mRNA Vaccination
Abstract
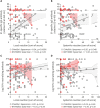
Correlation between vaccine reactogenicity and immunogenicity against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is unclear. Thus, we investigated to determine whether the reactogenicity after coronavirus disease 2019 vaccination is associated with antibody (Ab) titers and T cell responses. This study was prospective cohort study done with 131 healthcare workers at tertiary center in Seoul, South Korea. The degrees of the local reactions after the 1st and 2nd doses of ChAdOx1 nCov-19 (ChAdOx1) vaccination were significantly associated with the S1-specific IgG Ab titers (p=0.003 and 0.01, respectively) and neutralizing Ab (p=0.04 and 0.10, respectively) in age- and sex-adjusted multivariate analysis, whereas those after the BNT162b2 vaccination did not show significant associations. T cell responses did not show significant associations with the degree of reactogenicity after the ChAdOx1 vaccination or the BNT162b2 vaccination. Thus, high degree of local reactogenicity after the ChAdOx1 vaccine may be used as an indicator of strong humoral immune responses against SARS-CoV-2.
Keywords: Antibody response; COVID-19; Injection site reaction; Neutralizing antibody; Vaccine.
Copyright © 2021. The Korean Association of Immunologists.
Conflict of interest statement
Conflict of Interest: The authors declare no potential conflicts of interest.
Figures

References
-
- Kim SH, Bang JW, Park KH, Park WB, Kim HB, Kim NJ, Jee Y, Cho H, Oh MD, Choe KW. Prediction of residual immunity to smallpox, by means of an intradermal skin test with inactivated vaccinia virus. J Infect Dis. 2006;194:377–384. - PubMed
-
- Fenner F, Henderson DA, Arita I, Jezek Z, Ladnyi ID. Smallpox and its eradication. Geneva: World Health Organization; 1988.
-
- Wulff H, Chin TD, Wenner HA. Serologic responses of children after primary vaccination and revaccination against smallpox. Am J Epidemiol. 1969;90:312–318. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

