A Potential New Mouse Model of Axial Spondyloarthritis Involving the Complement System
- PMID: 35036032
- PMCID: PMC8733187
- DOI: 10.4110/in.2021.21.e45
A Potential New Mouse Model of Axial Spondyloarthritis Involving the Complement System
Abstract
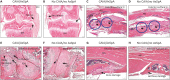
Many mouse models of rheumatoid arthritis have been identified, but only a limited number are present for axial spondyloarthritis (AxSpA). Collagen Ab-induced arthritis (CAIA) is one of the most widely used mouse models of arthritis, and it is complement-dependent. We found that mice developing CAIA also developed spinal lesions similar to those found in AxSpA. To induce CAIA, mice were injected intraperitoneally at day 0 with anti-collagen Abs, followed by LPS injection at day 3. CAIA mice demonstrated a significant kyphosis through the spine, as well as hypertrophic cartilage and osseous damage of the intravertebral joints. Immunohistochemical staining of the kyphotic area revealed increased complement C3 deposition and macrophage infiltration, with localization to the intravertebral joint margins. Near Infrared (NIR) in vivo imaging showed that anti-collagen Abs conjugated with IRDye® 800CW not only localized to cartilage surface in the joints but also to the spine in arthritic mice. We report here a novel preclinical mouse model in which, associated with the induction of CAIA, mice also exhibited salient features of AxSpA; this new experimental model of AxSpA may allow investigators to shed light on the local causal mechanisms of AxSpA bone and soft tissue changes as well as treatment.
Keywords: Arthritis; Axial spondyloarthritis; Complement.
Copyright © 2021. The Korean Association of Immunologists.
Conflict of interest statement
Conflict of Interest: The authors declare no potential conflicts of interest.
Figures



References
-
- Braun J, Bollow M, Remlinger G, Eggens U, Rudwaleit M, Distler A, Sieper J. Prevalence of spondylarthropathies in HLA-B27 positive and negative blood donors. Arthritis Rheum. 1998;41:58–67. - PubMed
-
- Tran TM, Dorris ML, Satumtira N, Richardson JA, Hammer RE, Shang J, Taurog JD. Additional human beta2-microglobulin curbs HLA-B27 misfolding and promotes arthritis and spondylitis without colitis in male HLA-B27-transgenic rats. Arthritis Rheum. 2006;54:1317–1327. - PubMed
-
- Baeten D, Breban M, Lories R, Schett G, Sieper J. Are spondylarthritides related but distinct conditions or a single disease with a heterogeneous phenotype? Arthritis Rheum. 2013;65:12–20. - PubMed
-
- Sherlock JP, Joyce-Shaikh B, Turner SP, Chao CC, Sathe M, Grein J, Gorman DM, Bowman EP, McClanahan TK, Yearley JH, et al. IL-23 induces spondyloarthropathy by acting on ROR-γt+ CD3+CD4−CD8− entheseal resident T cells. Nat Med. 2012;18:1069–1076. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

