Personalized therapy with peptide-based neoantigen vaccine (EVX-01) including a novel adjuvant, CAF®09b, in patients with metastatic melanoma
- PMID: 35036074
- PMCID: PMC8757480
- DOI: 10.1080/2162402X.2021.2023255
Personalized therapy with peptide-based neoantigen vaccine (EVX-01) including a novel adjuvant, CAF®09b, in patients with metastatic melanoma
Abstract
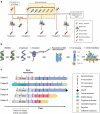
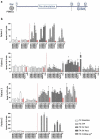
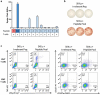
The majority of neoantigens arise from unique mutations that are not shared between individual patients, making neoantigen-directed immunotherapy a fully personalized treatment approach. Novel technical advances in next-generation sequencing of tumor samples and artificial intelligence (AI) allow fast and systematic prediction of tumor neoantigens. This study investigates feasibility, safety, immunity, and anti-tumor potential of the personalized peptide-based neoantigen vaccine, EVX-01, including the novel CD8+ T-cell inducing adjuvant, CAF®09b, in patients with metastatic melanoma (NTC03715985). The AI platform PIONEERTM was used for identification of tumor-derived neoantigens to be included in a peptide-based personalized therapeutic cancer vaccine. EVX-01 immunotherapy consisted of 6 administrations with 5-10 PIONEERTM-predicted neoantigens as synthetic peptides combined with the novel liposome-based Cationic Adjuvant Formulation 09b (CAF®09b) to strengthen T-cell responses. EVX-01 was combined with immune checkpoint inhibitors to augment the activity of EVX-01-induced immune responses. The primary endpoint was safety, exploratory endpoints included feasibility, immunologic and objective responses. This interim analysis reports the results from the first dose-level cohort of five patients. We documented a short vaccine manufacturing time of 48-55 days which enabled the initiation of EVX-01 treatment within 60 days from baseline biopsy. No severe adverse events were observed. EVX-01 elicited long-lasting EVX-01-specific T-cell responses in all patients. Competitive manufacturing time was demonstrated. EVX-01 was shown to be safe and able to elicit immune responses targeting tumor neoantigens with encouraging early indications of a clinical and meaningful antitumor efficacy, warranting further study.
Keywords: Personalized therapy; cancer vaccine; immune response; immunotherapy; neoantigen.
© 2022 The Author(s). Published with license by Taylor & Francis Group, LLC.
Conflict of interest statement
Marco Donia has received honoraria for lectures from Roche and Novartis (past 2 years). Inge Marie Svane has received honoraria for consultancies and lectures from Novartis, Roche, MSD, Pierre Fabre, and BMS. CCIT-DK has received economic support for trial personal wages from Evaxion Biotech A/S, Denmark. SRH is the cofounder of Tetramer-shop and PokeACell and is the co-inventor of a number of licensed patents. ABS, TT, CG, JFN and JVK are employees of Evaxion Biotech A/S and have financial interest in the company. All other authors declare that they have no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
