High-Dose Dexamethasone Versus Tocilizumab in Moderate to Severe COVID-19 Pneumonia: A Randomized Controlled Trial
- PMID: 35036193
- PMCID: PMC8752381
- DOI: 10.7759/cureus.20353
High-Dose Dexamethasone Versus Tocilizumab in Moderate to Severe COVID-19 Pneumonia: A Randomized Controlled Trial
Abstract
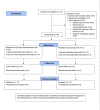
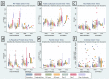
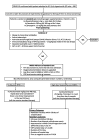
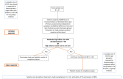
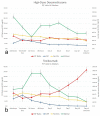
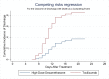
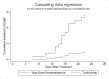
Background and objectives Recent randomized controlled trials (RCTs) have indicated potential therapeutic benefits with high-dose dexamethasone (HDD) or tocilizumab (TCZ) plus standard care in moderate to severe coronavirus disease 2019 (COVID-19) with acute respiratory distress syndrome (ARDS). No study has compared these two against each other. We aimed to compare the efficacy and safety of HDD against TCZ in moderate to severe COVID-ARDS. Methods Patients admitted with moderate to severe COVID-19 ARDS with clinical worsening within 48 hours of standard care were randomly assigned to receive either HDD or TCZ plus standard care. The primary outcome was ventilator-free days (VFDs) at 28 days. The main secondary outcomes were 28-day all-cause mortality and the incidence of adverse events. Our initial plan was to perform an interim analysis of the first 42 patients. Results VFDs were significantly lower in the HDD arm (median difference: 28 days; 95% confidence interval (CI): 19.35-36.65; Cohen's d = 1.14;p < 0.001). We stopped the trial at the first interim analysis due to high 28-day mortality in the HDD arm (relative risk (RR) of death: 6.5; p = 0.007; NNT (harm) = 1.91). The incidence of secondary infections was also significantly high in the HDD arm (RR: 5.5; p = 0.015; NNT (harm) = 2.33). Conclusions In our study population, HDD was associated with a very high rate of mortality and adverse events. We would not recommend HDD to mitigate the cytokine storm in moderate to severe COVID-19 ARDS. TCZ appears to be a much better and safer alternative.
Keywords: acute respiratory distress syndrome (ards); covid-19; cytokine storms; fungal infection; high-dose dexamethasone; pulse dose steroids; secondary infection; tocilizumab.
Copyright © 2021, Naik et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. JAMA. 2020;323:1824–1836. - PubMed
LinkOut - more resources
Full Text Sources
