Validation of mailed via postal service dried blood spot cards on commercially available HIV testing systems
- PMID: 35036621
- PMCID: PMC8692097
- DOI: 10.35772/ghm.2021.01105
Validation of mailed via postal service dried blood spot cards on commercially available HIV testing systems
Abstract
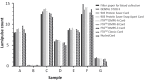
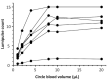
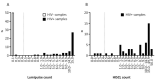
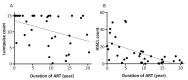
The demand for HIV testing using dried blood spots (DBS) has increased recently. However, DBS is not an approved sample for HIV testing in Japan. This study examined the validation of HIV testing with DBS, prepared at the laboratory or remotely and mailed via postal service to the laboratory. DBS were punched out from a 5.5 mm diameter circle on filter paper, then eluted with 600 μL of phosphate buffered saline overnight at 4℃, and analyzed by Lumipulse S HIVAg/Ab (LUM). The mean LUM count of DBS was 237.4-times diluted compared to titrated plasma. Repeated sample testing showed that although LUM count of DBS decreased slightly with increase in sample storage time (up to one month), it did not affect the result of HIV testing with DBS. Based on testing of 50 HIV+ confirmed cases and 50 HIV- persons, the estimated sensitivity was 98% (49/50) with a specificity of 100% when the cut-off value is 0.5. The single false negative case was a patient with undetectable viral load over the last 10 years, resulting in a decrease of antibody titer below the cut-off level. In conclusion, although DBS cannot completely replace plasma in HIV testing because the sensitivity was a little lower than that of plasma, it can be potentially useful for a screening test by self-finger-prick and postal service use. This will allow people to receive HIV testing without visiting public health centers.
Keywords: HIV; dried blood spot; postal service; self-finger-prick.
2021, National Center for Global Health and Medicine.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures

Similar articles
-
Assessment of HIV prevalence among MSM in Tokyo using self-collected dried blood spots delivered through the postal service.BMC Infect Dis. 2018 Dec 5;18(1):627. doi: 10.1186/s12879-018-3491-0. BMC Infect Dis. 2018. PMID: 30518333 Free PMC article.
-
Are all blood-based postal sampling kits the same? A comparative service evaluation of the performance of dried blood spot and mini tube sample collection systems for postal HIV and syphilis testing.Sex Transm Infect. 2021 May;97(3):209-214. doi: 10.1136/sextrans-2020-054692. Epub 2020 Nov 19. Sex Transm Infect. 2021. PMID: 33214322
-
Dried blood spot and mini-tube blood sample collection kits for postal HIV testing services: a comparative review of successes in a real-world setting.Sex Transm Infect. 2019 Feb;95(1):43-45. doi: 10.1136/sextrans-2018-053567. Epub 2018 Aug 2. Sex Transm Infect. 2019. PMID: 30072393
-
Dried blood spots for HIV-1 drug resistance and viral load testing: A review of current knowledge and WHO efforts for global HIV drug resistance surveillance.AIDS Rev. 2010 Oct-Dec;12(4):195-208. AIDS Rev. 2010. PMID: 21179184 Review.
-
The use of mass spectrometry to analyze dried blood spots.Mass Spectrom Rev. 2016 May-Jun;35(3):361-438. doi: 10.1002/mas.21441. Epub 2014 Sep 22. Mass Spectrom Rev. 2016. PMID: 25252132 Review.
Cited by
-
Feasibility of community at-home dried blood spot collection combined with pooled reverse transcription PCR as a viable and convenient method for malaria epidemiology studies.Malar J. 2022 Jul 14;21(1):221. doi: 10.1186/s12936-022-04239-x. Malar J. 2022. PMID: 35836179 Free PMC article.
-
Rethinking detection of pre-existing and intervening Plasmodium infections in malaria clinical trials.Front Immunol. 2022 Sep 20;13:1003452. doi: 10.3389/fimmu.2022.1003452. eCollection 2022. Front Immunol. 2022. PMID: 36203582 Free PMC article. Review.
References
-
- AIDS Prevention Information Network, Japan Foundation for AIDS Prevention. Number of HIV testing in local health centers, annual report for trends in AIDS. https://api-net.jfap.or.jp/status/japan/data/2019/nenpo/kensa.pdf (accessed June 26, 2021). (in Japanese) .
-
- World Health Organization. Consolidated guidelines on HIV testing services 2019. https://www.who.int/publications/i/item/978-92-4-155058-1 (accessed June 25, 2021).
-
- Bertagnolio S, Parkin NT, Jordan M, Brooks J, Garcia- Lerma JG. Dried blood spots for HIV-1 drug resistance and viral load testing. A review of current knowledge and WHO efforts for global HIV drug resistance surveillance. AIDS Rev. 2010; 12:195-208 - PubMed
-
- Chang J, de Sousa A, Sabatier J, Assane M, Zhang G, Bila D, Vaz P, Alfredo C, Cossa L, Bhatt N, Koumans EH, Yang C, Rivadeneira E, Jani I, Houston JC. Performance characteristics of finger-stick dried blood spots (DBS) on the determination of human immunodeficiency virus (HIV) treatment failure in a pediatric population in Mozambique. PLoS One. 2017; 12:e0181054. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

