Efficient Preparation of a Nonenzymatic Nanoassembly Based on Cobalt-Substituted Polyoxometalate and Polyethylene Imine-Capped Silver Nanoparticles for the Electrochemical Sensing of Carbofuran
- PMID: 35036686
- PMCID: PMC8757336
- DOI: 10.1021/acsomega.1c04198
Efficient Preparation of a Nonenzymatic Nanoassembly Based on Cobalt-Substituted Polyoxometalate and Polyethylene Imine-Capped Silver Nanoparticles for the Electrochemical Sensing of Carbofuran
Abstract
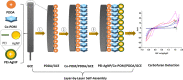
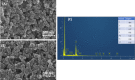
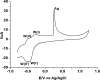
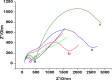
The ever-growing exploitation of pesticides and their lethal effects on living beings have made it a dire need of the day to develop an accurate and reliable approach for their monitoring at trace levels. The designing of an enzyme-free electrocatalyst to electrochemically detect the pesticide residues is currently gaining much importance. In this study, a novel redox-sensing film was constructed successfully based on cobalt-substituted Dawson-type polyoxometalate [P2W17O61 (Co2+·OH2)]7- (Co-POM) and polyethylene imine (PEI)-capped silver nanoparticles (AgNPs). A nanohybrid assembly was fabricated on a glassy carbon electrode's surface by alternately depositing Co-POM and PEI-AgNPs using the layer-by-layer self-assembly method. The surface morphology of the immobilized CoPOM/AgNP multilayer nanoassembly was analyzed through scanning electron microscopy along with energy-dispersive spectroscopy for elemental analysis. The redox properties and surface morphologies of fabricated assemblies were evaluated by cyclic voltammetry and electrochemical impedance spectroscopy. The practicability and feasibility of the proposed sensing layer was tested for the detection of a highly toxic insecticide, that is, carbofuran. The fabricated sensor exhibited a limit of detection of 0.1 mM with a sensitivity of 13.11 μA mM-1 for carbofuran. The results depicted that the fabricated nonenzymatic hybrid film showed excellent electrocatalytic efficiency for the carbofuran oxidation. Furthermore, the obtained value of "apparent Km", that is, 0.4 mM, illustrates a good electro-oxidation activity of the sensor for the detection of carbofuran. The exceptionally stable redox activity of Co-POM, high surface area and greater conductivity of AgNPs, and the synergistic effect of all components of the film resulted in an excellent analytical performance of the proposed sensing assembly. This work provides a new direction to the progress and designing of nonenzymatic electrochemical sensors for pesticide determination in real samples.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
-
- USEPA . Setting Tolerances for Pesticide Residues in Foods, 2016.
-
- Ramachandran R.; Chen T.-W.; Chen S.-M.; Baskar T.; Kannan R.; Elumalai P.; Raja P.; Jeyapragasam T.; Dinakaran K.; Gnana kumar G. p. A review of the advanced developments of electrochemical sensors for the detection of toxic and bioactive molecules. Inorg. Chem. 2019, 6, 3418–3439. 10.1039/c9qi00602h. - DOI
-
- Tanwar S.; Mathur D. J. Graphene-based nanocomposites as sensing elements for the electrochemical detection of pesticides: a review. J. Solid State Electrochem. 2021, 25, 2145–2159. 10.1007/s10008-021-04990-2. - DOI
LinkOut - more resources
Full Text Sources

