TfNN15N: A γ-15N-Labeled Diazo-Transfer Reagent for the Synthesis of β-15N-Labeled Azides
- PMID: 35036700
- PMCID: PMC8757338
- DOI: 10.1021/acsomega.1c04679
TfNN15N: A γ-15N-Labeled Diazo-Transfer Reagent for the Synthesis of β-15N-Labeled Azides
Abstract
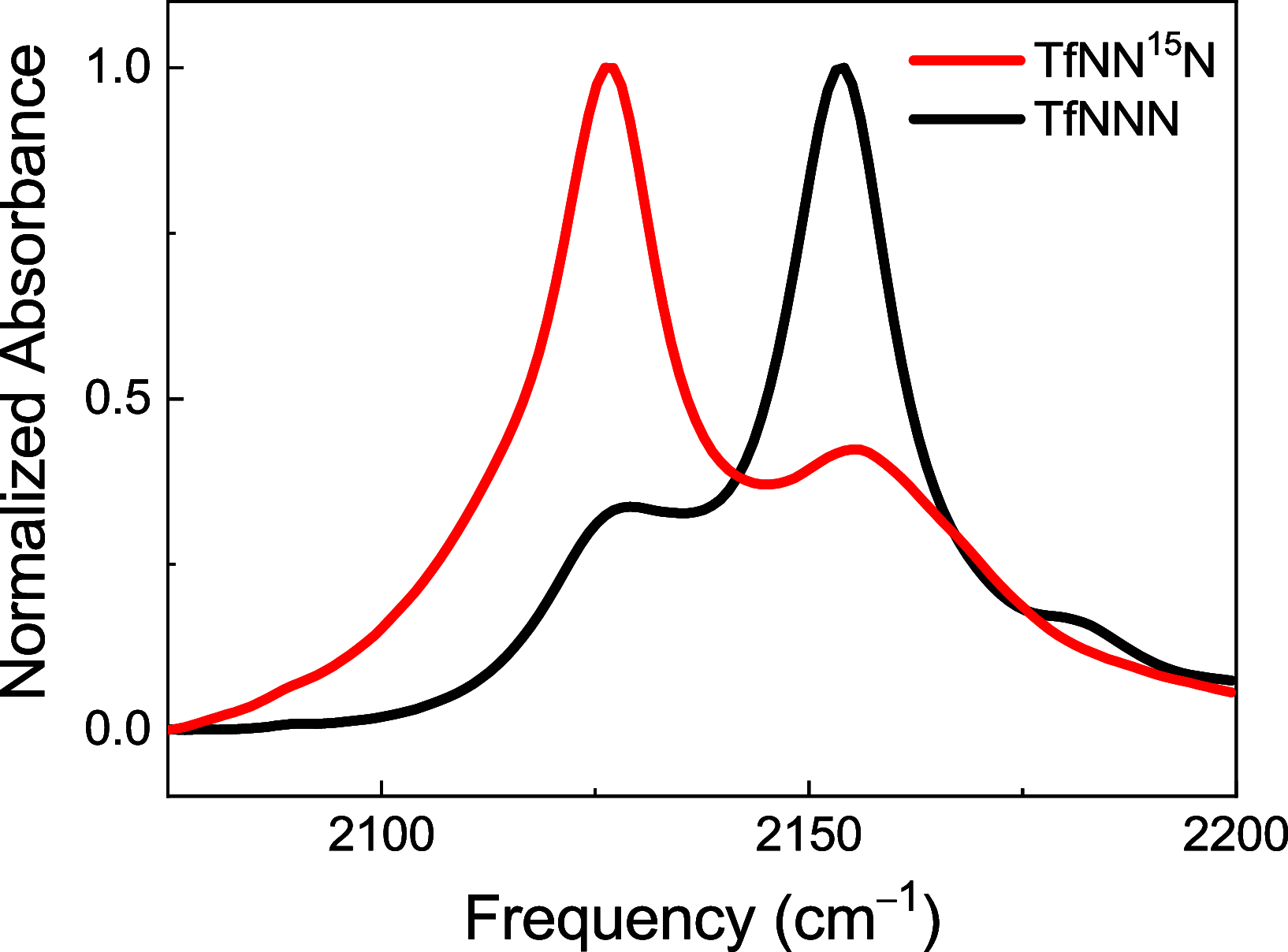
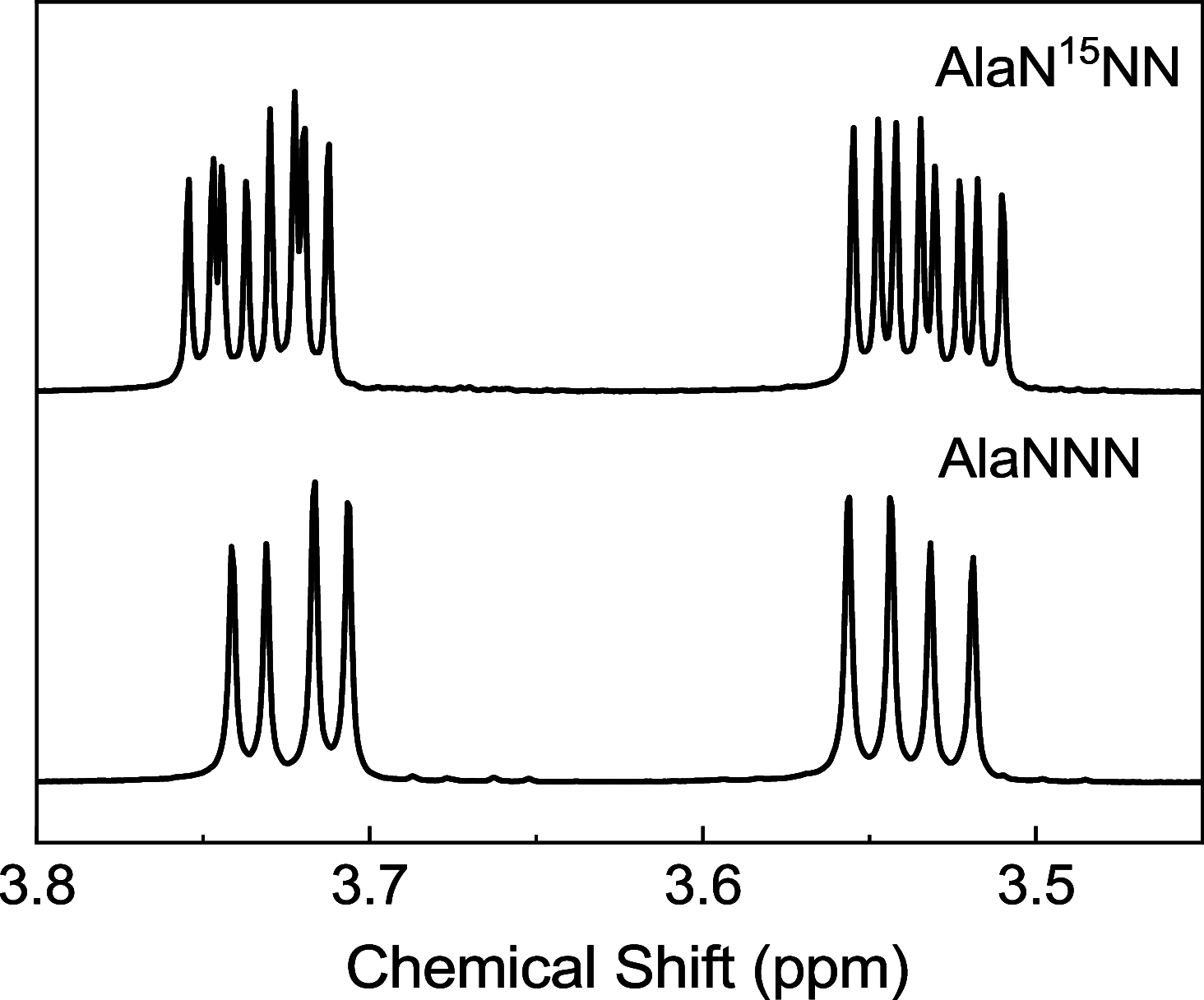
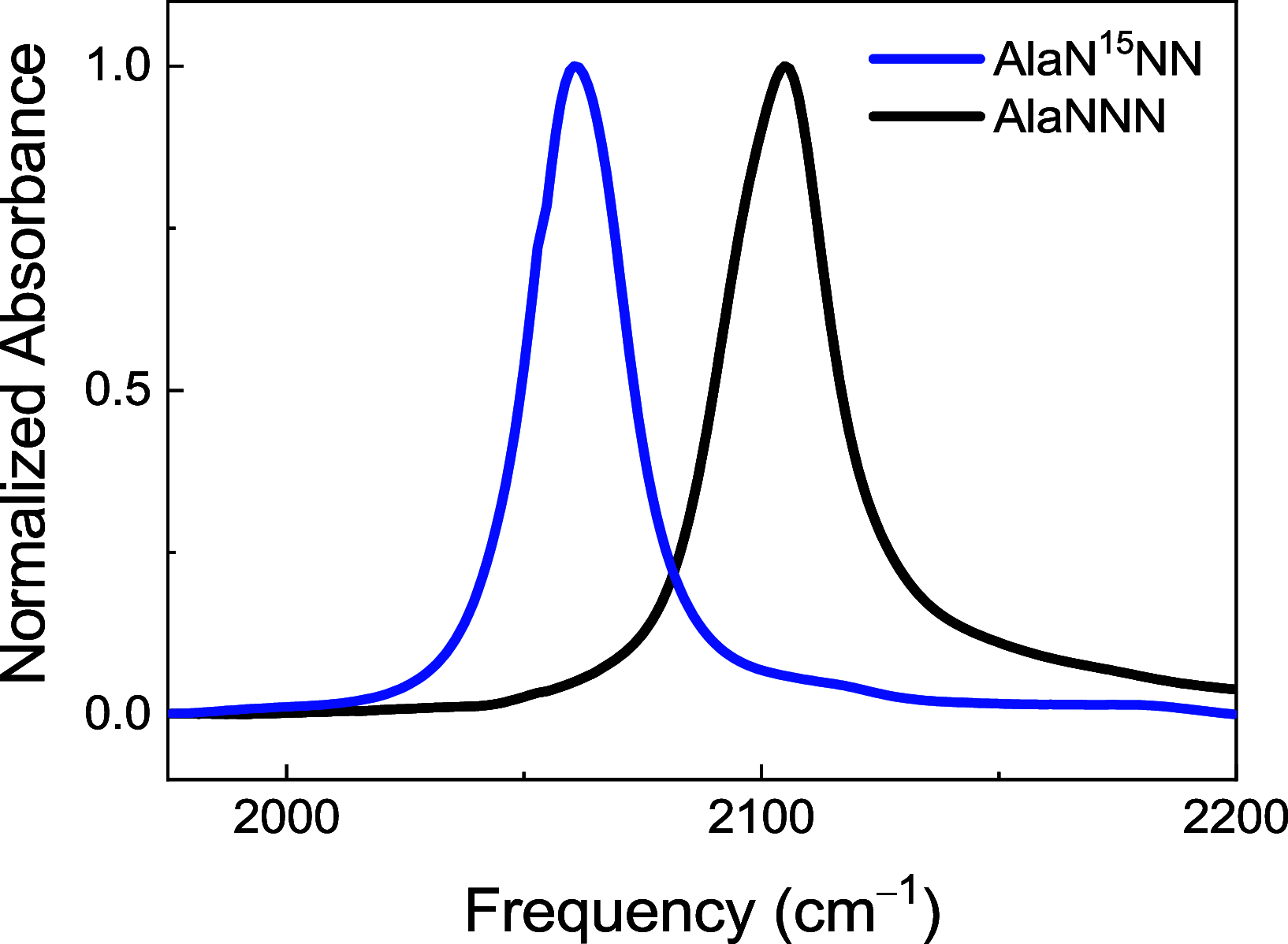
Azides are infrared (IR) probes that are important for structure and dynamics studies of proteins. However, they often display complex IR spectra owing to Fermi resonances and multiple conformers. Isotopic substitution of azides weakens the Fermi resonance, allowing more accurate IR spectral analysis. Site-specifically 15N-labeled aromatic azides, but not aliphatic azides, are synthesized through nitrosation. Both 15N-labeled aromatic and aliphatic azides are synthesized through nucleophilic substitution or diazo-transfer reaction but as an isotopomeric mixture. We present the synthesis of TfNN15N, a γ-15N-labeled diazo-transfer reagent, and its use to prepare β-15N-labeled aliphatic as well as aromatic azides.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Efficient and Inexpensive Synthesis of 15N-Labeled 2-Azido-1,3-dimethylimidazolinium Salts Using Na15NO2 Instead of Na15NNN.ACS Omega. 2024 Jan 30;9(6):6556-6560. doi: 10.1021/acsomega.3c07147. eCollection 2024 Feb 13. ACS Omega. 2024. PMID: 38371833 Free PMC article.
-
Two-dimensional IR spectroscopy reveals a hidden Fermi resonance band in the azido stretch spectrum of β-azidoalanine.Phys Chem Chem Phys. 2020 Sep 8;22(34):19223-19229. doi: 10.1039/d0cp02693j. Phys Chem Chem Phys. 2020. PMID: 32812969
-
Nitrosation with Sodium Hexanitrocobaltate(III).J Org Chem. 1997 Oct 17;62(21):7165-7169. doi: 10.1021/jo9703820. J Org Chem. 1997. PMID: 11671821
-
A reagent for safe and efficient diazo-transfer to primary amines: 2-azido-1,3-dimethylimidazolinium hexafluorophosphate.Org Biomol Chem. 2014 Jul 7;12(25):4397-406. doi: 10.1039/c4ob00515e. Org Biomol Chem. 2014. PMID: 24840505
-
Azidoimidazolinium Salts: Safe and Efficient Diazo-transfer Reagents and Unique Azido-donors.Chem Rec. 2017 Jul;17(7):653-666. doi: 10.1002/tcr.201600118. Epub 2016 Dec 21. Chem Rec. 2017. PMID: 28000372 Review.
Cited by
-
Efficient and Inexpensive Synthesis of 15N-Labeled 2-Azido-1,3-dimethylimidazolinium Salts Using Na15NO2 Instead of Na15NNN.ACS Omega. 2024 Jan 30;9(6):6556-6560. doi: 10.1021/acsomega.3c07147. eCollection 2024 Feb 13. ACS Omega. 2024. PMID: 38371833 Free PMC article.
References
-
- Lockhart D. J.; Kim P. S. Internal Stark effect measurement of the electric field at the amino terminus of an α helix. Science 1992, 257, 947–951. 10.1126/science.1502559. - DOI - PubMed
- Lockhart D. J.; Kim P. S. Electrostatic screening of charge and dipole interactions with the helix backbone. Science 1993, 260, 198–202. 10.1126/science.8469972. - DOI - PubMed
- Giaimo J. M.; Gusev A. V.; Wasielewski M. R. Excited-state symmetry breaking in cofacial and linear dimers of a green perylenediimide chlorophyll analogue leading to ultrafast charge separation. J. Am. Chem. Soc. 2002, 124, 8530–8531. 10.1021/ja026422l. - DOI - PubMed
- Miyawaki A.; Llopis J.; Heim R.; McCaffery J. M.; Adams J. A.; Ikural M.; Tsien R. Y. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature 1997, 388, 882–887. 10.1038/42264. - DOI - PubMed
- Tsien R. Y. The green fluorescent protein. Annu. Rev. Biochem. 1998, 67, 509–544. 10.1146/annurev.biochem.67.1.509. - DOI - PubMed
-
- Park E. S.; Boxer S. G. Origins of the sensitivity of molecular vibrations to electric fields: Carbonyl and nitrosyl stretches in model compounds and proteins. J. Phys. Chem. B 2002, 106, 5800–5806. 10.1021/jp0203043. - DOI
- Stavrov S. S.; Wright W. W.; Vanderkooi J. M.; Fidy J.; Kaposi A. D. Optical and IR absorption as probe of dynamics of heme proteins. Biopolymers 2002, 67, 255–258. 10.1002/bip.10103. - DOI - PubMed
-
- Chattopadhyay A.; Boxer S. G. Vibrational Stark effect spectroscopy. J. Am. Chem. Soc. 1995, 117, 1449–1450. 10.1021/ja00109a038. - DOI
- Schultz K. C.; Supekova L.; Ryu Y.; Xie J.; Perera R.; Schultz P. G. A genetically encoded infrared probe. J. Am. Chem. Soc. 2006, 128, 13984–13985. 10.1021/ja0636690. - DOI - PubMed
-
- Fafarman A. T.; Webb L. J.; Chuang J. I.; Boxer S. G. Site-specific conversion of cysteine thiols into thiocyanate creates an IR probe for electric fields in proteins. J. Am. Chem. Soc. 2006, 128, 13356–13357. 10.1021/ja0650403. - DOI - PMC - PubMed
- Park K.-H.; Jeon J.; Park Y.; Lee S.; Kwon H.-J.; Joo C.; Park S.; Han H.; Cho M. Infrared probes based on nitrile-derivatized prolines: Thermal insulation effect and enhanced dynamic range. J. Phys. Chem. Lett. 2013, 4, 2105–2110. 10.1021/jz400954r. - DOI
-
- Adhikary R.; Zimmermann J.; Romesberg F. E. Transparent window vibrational probes for the characterization of proteins with high structural and temporal resolution. Chem. Rev. 2017, 117, 1927–1969. 10.1021/acs.chemrev.6b00625. - DOI - PubMed
- Chin J. K.; Jimenez R.; Romesberg F. E. Direct observation of protein vibrations by selective incorporation of spectroscopically observable carbon-deuterium bonds in cytochrome c. J. Am. Chem. Soc. 2001, 123, 2426–2427. 10.1021/ja0033741. - DOI - PubMed
- Romesberg F. E. Multidisciplinary experimental approaches to characterizing biomolecular dynamics. ChemBioChem 2003, 4, 563–571. 10.1002/cbic.200300572. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Medical

