Identification, Characterization, and Expression Analysis of Carotenoid Biosynthesis Genes and Carotenoid Accumulation in Watercress (Nasturtium officinale R. Br.)
- PMID: 35036712
- PMCID: PMC8756599
- DOI: 10.1021/acsomega.1c04802
Identification, Characterization, and Expression Analysis of Carotenoid Biosynthesis Genes and Carotenoid Accumulation in Watercress (Nasturtium officinale R. Br.)
Abstract
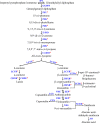
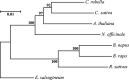
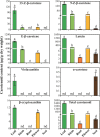
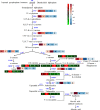
Watercress (Nasturtium officinale R. Br.) is an important aquatic herb species belonging to the Brassicaceae family. It has various medicinal properties and has been utilized for the treatment of cancer and other diseases; however, currently available genomic information regarding this species is limited. Here, we performed the first comprehensive analysis of the carotenoid biosynthesis pathway (CBP) genes of N. officinale, which were identified from next-generation sequencing data. We identified and characterized 11 putative carotenoid pathway genes; among these, nine full and two partial open reading frames were determined. These genes were closely related to CBP genes of the other higher plants in the phylogenetic tree. Three-dimensional structure analysis and multiple alignments revealed several distinct conserved motifs, including aspartate or glutamate residues, carotene-binding motifs, and dinucleotide-binding motifs. Quantitative reverse transcription-polymerase chain reaction results showed that the CBP was expressed in a tissue-specific manner: expression levels of NoPSY, NoPDS, NoZDS-p, NoCrtISO, NoLCYE, NoCHXE-p, and NoCCD were highest in the flower, whereas NoLCYB, NoCHXB, NoZEP, and NoNCED were highest in the leaves. Stems, roots, and seeds did not show a significant change in the expression compared to the leaves and flowers. High-performance liquid chromatography analysis of the same organs showed the presence of seven distinct carotenoid compounds. The total carotenoid content was highest in the leaves followed by flowers, seeds, stems, and roots. Among the seven individual carotenoids, the levels of six carotenoids (i.e., 13-Z-β-carotene, 9-Z-β-carotene, E-β-carotene, lutein, violaxanthin, and β-cryptoxanthin) were highest in the leaves. The highest content was observed for lutein, followed by E-β-carotene, and 9-Z-β-carotene; these carotenoids were much higher in the leaves compared to the other organs. The results will be useful references for further molecular genetics and functional studies involving this species and other closely related species.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
Identification, In Silico Characterization, and Differential Expression Profiles of Carotenoid, Xanthophyll, Apocarotenoid Biosynthetic Pathways Genes, and Analysis of Carotenoid and Xanthophyll Accumulation in Heracleum moellendorffii Hance.Int J Mol Sci. 2022 Apr 27;23(9):4845. doi: 10.3390/ijms23094845. Int J Mol Sci. 2022. PMID: 35563233 Free PMC article.
-
Molecular Characterization, Expression Analysis of Carotenoid, Xanthophyll, Apocarotenoid Pathway Genes, and Carotenoid and Xanthophyll Accumulation in Chelidonium majus L.Plants (Basel). 2021 Aug 23;10(8):1753. doi: 10.3390/plants10081753. Plants (Basel). 2021. PMID: 34451798 Free PMC article.
-
Identification and analysis of phenylpropanoid biosynthetic genes and phenylpropanoid accumulation in watercress (Nasturtium officinale R. Br.).3 Biotech. 2020 Jun;10(6):260. doi: 10.1007/s13205-020-02244-y. Epub 2020 May 19. 3 Biotech. 2020. PMID: 32477847 Free PMC article.
-
De novo transcriptome analysis and glucosinolate profiling in watercress (Nasturtium officinale R. Br.).BMC Genomics. 2017 May 23;18(1):401. doi: 10.1186/s12864-017-3792-5. BMC Genomics. 2017. PMID: 28535746 Free PMC article.
-
Transcriptional Regulation of Carotenoid Biosynthesis in Plants: So Many Regulators, So Little Consensus.Front Plant Sci. 2019 Aug 9;10:1017. doi: 10.3389/fpls.2019.01017. eCollection 2019. Front Plant Sci. 2019. PMID: 31447877 Free PMC article. Review.
Cited by
-
Comparative Genomic Analysis of Sphingomonas morindae sp. NBD5 and Sphingopyxis sp. USTB-05 for Producing Macular Pigment.Microorganisms. 2023 Jan 19;11(2):266. doi: 10.3390/microorganisms11020266. Microorganisms. 2023. PMID: 36838230 Free PMC article.
-
Metabolic Profiling of Primary and Secondary Metabolites in Kohlrabi (Brassica oleracea var. gongylodes) Sprouts Exposed to Different Light-Emitting Diodes.Plants (Basel). 2023 Mar 13;12(6):1296. doi: 10.3390/plants12061296. Plants (Basel). 2023. PMID: 36986982 Free PMC article.
-
Identification, In Silico Characterization, and Differential Expression Profiles of Carotenoid, Xanthophyll, Apocarotenoid Biosynthetic Pathways Genes, and Analysis of Carotenoid and Xanthophyll Accumulation in Heracleum moellendorffii Hance.Int J Mol Sci. 2022 Apr 27;23(9):4845. doi: 10.3390/ijms23094845. Int J Mol Sci. 2022. PMID: 35563233 Free PMC article.
-
Preventive and therapeutic effects of Nasturtium officinale hydroalcoholic extract on cyclophosphamide-induced testicular toxicity in rats.Avicenna J Phytomed. 2025 May-Jun;15(3):1091-1101. doi: 10.22038/ajp.2025.25887. Avicenna J Phytomed. 2025. PMID: 40365180 Free PMC article.
-
Carotenoid metabolomic and transcriptomic analyses provide insights into the flower color transition in Lonicera macranthoides.BMC Biotechnol. 2025 Jul 9;25(1):69. doi: 10.1186/s12896-025-01007-y. BMC Biotechnol. 2025. PMID: 40634871 Free PMC article.
References
-
- Cruz R. M. S.; Vieira M. C.; Silva C. L. M. Effect of heat and thermosonication treatments on watercress (Nasturtium officinale) vitamin C degradation kinetics. Innov. Food Sci. Emerg. 2008, 9, 483–488. 10.1016/j.ifset.2007.10.005. - DOI
-
- Pourhassan-Moghaddam M.; Zarghami N.; Mohsenifar A.; Rahmati-Yamchi M.; Gholizadeh D.; Akbarzadeh A.; de la Guardia M.; Nejati-Koshki K. Watercress-based gold nanoparticles: biosynthesis, mechanism of formation and study of their biocompatibility in vitro. Micro. Nano Lett. 2014, 9, 345–350. 10.1049/mnl.2014.0063. - DOI
-
- Gonçalves E. M.; Cruz R. M. S.; Abreu M.; Brandão T. R. S.; Silva C. L. M. Biochemical and colour changes of watercress (Nasturtium officinale R. Br.) during freezing and frozen storage. J. Food Eng. 2009, 93, 32–39. 10.1016/j.jfoodeng.2008.12.027. - DOI
LinkOut - more resources
Full Text Sources

