Biophysical and Structural Characterization of Ribulose-5-phosphate Epimerase from Leishmania donovani
- PMID: 35036723
- PMCID: PMC8756792
- DOI: 10.1021/acsomega.1c04967
Biophysical and Structural Characterization of Ribulose-5-phosphate Epimerase from Leishmania donovani
Abstract
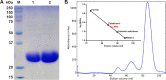
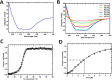
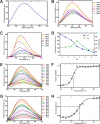
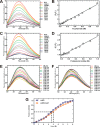
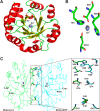
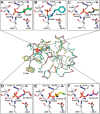
Pentose phosphate pathway (PPP) plays a crucial role in the maintenance of NADPH/NADP+ homeostasis and provides protection against oxidative stress through detoxification of the reactive oxygen species. Ribulose-5-phosphate epimerase (RPE) participates in catalysis of the interconversion of ribulose-5-phosphate (Ru5P) to xylulose-5-phosphate (Xu5P) during PPP, however the structural attributes of this enzyme are still underexplored in many human pathogens including leishmanial parasites. The present study focuses upon cloning, purification and characterization of RPE of Leishmania donovani (LdRPE) using various biophysical and structural approaches. Sequence analysis has shown the presence of trypanosomatid-specific insertions at the N-terminus that are absent in humans and other eukaryotes. Gel filtration chromatography indicated recombinant LdRPE to exist as a dimer in the solution. Circular dichroism studies revealed a higher alpha helical content at physiological pH and temperature that comparatively varies with changing these parameters. Additionally, intrinsic fluorescence and quenching studies of LdRPE have depicted that tryptophan residues are mainly buried in the hydrophobic regions, and the recombinant enzyme is moderately tolerant to urea. Moreover, homology modeling was employed to generate the three-dimensional structure of LdRPE followed by molecular docking with the substrate, product, and substrate analogues. The modeled structure of LdRPE unravelled the presence of conserved active site residues as well as a single binding pocket for the substrate and product, while an in silico study suggested binding of substrate analogues into a similar pocket with more affinity than the substrate. Additionally, molecular dynamics simulation analysis has deciphered complexes of LdRPE with most of the ligands exhibiting more stability than its apo form and lesser fluctuations in active site residues in the presence of ligands. Altogether, our study presents structural insights into leishmanial RPE that could provide the basis for its implication to develop potent antileishmanials.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
Biochemical and structural insights into 6-phosphogluconate dehydrogenase from Leishmania donovani.Appl Microbiol Biotechnol. 2021 Jul;105(13):5471-5489. doi: 10.1007/s00253-021-11434-4. Epub 2021 Jul 12. Appl Microbiol Biotechnol. 2021. PMID: 34250571
-
Conversion of D-ribulose 5-phosphate to D-xylulose 5-phosphate: new insights from structural and biochemical studies on human RPE.FASEB J. 2011 Feb;25(2):497-504. doi: 10.1096/fj.10-171207. Epub 2010 Oct 5. FASEB J. 2011. PMID: 20923965 Free PMC article.
-
Characterization of chloroplast ribulose-5-phosphate-3-epimerase from the microalga Chlamydomonas reinhardtii.Plant Physiol. 2024 Mar 29;194(4):2263-2277. doi: 10.1093/plphys/kiad680. Plant Physiol. 2024. PMID: 38134324
-
D-Ribulose 5-phosphate 3-epimerase: functional and structural relationships to members of the ribulose-phosphate binding (beta/alpha)8-barrel superfamily.Biochemistry. 2006 Feb 28;45(8):2493-503. doi: 10.1021/bi052474m. Biochemistry. 2006. PMID: 16489742
-
Proliferating tumor cells mimick glucose metabolism of mature human erythrocytes.Cell Cycle. 2019 Jun;18(12):1316-1334. doi: 10.1080/15384101.2019.1618125. Epub 2019 Jun 3. Cell Cycle. 2019. PMID: 31154896 Free PMC article. Review.
Cited by
-
Metabolomics analysis of visceral leishmaniasis based on urine of golden hamsters.Parasit Vectors. 2023 Aug 30;16(1):304. doi: 10.1186/s13071-023-05881-3. Parasit Vectors. 2023. PMID: 37649093 Free PMC article.
-
Transcriptome analysis reveals the effect of cold storage time on the expression of genes related to oxidative metabolism in Chinese black truffle.Front Nutr. 2024 Jun 4;11:1375386. doi: 10.3389/fnut.2024.1375386. eCollection 2024. Front Nutr. 2024. PMID: 38895661 Free PMC article.
-
Playbook workflow builder: Interactive construction of bioinformatics workflows.PLoS Comput Biol. 2025 Apr 3;21(4):e1012901. doi: 10.1371/journal.pcbi.1012901. eCollection 2025 Apr. PLoS Comput Biol. 2025. PMID: 40179105 Free PMC article.
References
-
- Stincone A.; Prigione A.; Cramer T.; Wamelink M. M. C.; Campbell K.; Cheung E.; Olin-Sandoval V.; Grüning N. M.; Krüger A.; Tauqeer Alam M.; Keller M. A.; Breitenbach M.; Brindle K. M.; Rabinowitz J. D.; Ralser M. The return of metabolism: biochemistry and physiology of the pentose phosphate pathway. Biol. Rev. Cambridge Philos. Soc. 2015, 90, 927–963. 10.1111/brv.12140. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

