Reliable measurement of plasma kinin peptides: Importance of preanalytical variables
- PMID: 35036825
- PMCID: PMC8753134
- DOI: 10.1002/rth2.12646
Reliable measurement of plasma kinin peptides: Importance of preanalytical variables
Abstract
Background: The kallikrein-kinin system is involved in many (patho)physiological processes and kinin peptides are considered potential clinical biomarkers. Variance in blood specimen collection and processing, artificial ex vivo bradykinin formation, and rapid degradation of kinins have contributed to divergence in published plasma levels, therefore limiting their significance. Thus, reliable preanalytical settings are highly required.
Objectives: This study aimed to develop and evaluate a standardized preanalytical procedure for reliable kinin quantification. The procedure was based on identification of the most impactful variables on ex vivo plasma level alterations.
Methods: Suitable protease inhibitors and blood specimen collection and handling conditions were systematically investigated. Their influence on plasma levels of seven kinins was monitored using an established in-house liquid chromatography-tandem mass spectrometry platform.
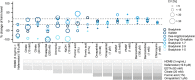
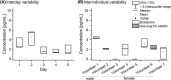
Results: In nonstandardized settings, ex vivo rise of bradykinin was found to already occur 30 seconds after blood sampling with high interindividual variation. The screening of 17 protease inhibitors resulted in a customized seven-component protease inhibitor, which efficiently stabilized ex vivo kinin levels. The reliability of kinin levels was substantially jeopardized by prolonged rest time until centrifugation, phlebotomy methodology (eg, straight needles, catheters), vacuum sampling technique, or any time delays during venipuncture. The subsequently developed standardized procedure was applied to healthy volunteers and proved it significantly limited interday and interindividual kinin level variability.
Conclusion: The developed procedure for blood specimen collection and handling is feasible in clinical settings and allows for determination of reliable kinin levels. It may contribute to further elucidating the role of the kallikrein-kinin system in diseases like angioedema, sepsis, or coronavirus disease 2019.
Keywords: blood specimen collection; bradykinin; factor XII; kallikrein‐kinin system; phlebotomy.
© 2022 The Authors. Research and Practice in Thrombosis and Haemostasis published by Wiley Periodicals LLC on behalf of International Society on Thrombosis and Haemostasis (ISTH).
Figures





References
-
- Campbell DJ. Bradykinin Peptides. In: Kastin AJ, editor. Handbook of biologically active peptides, 2nd edn. Elsevier; 2013. p. 1386‐1393.
-
- Kashuba E, Bailey J, Allsup D, Cawkwell L. The kinin‐kallikrein system: physiological roles, pathophysiology and its relationship to cancer biomarkers. Biomarkers. 2013;18:279‐296. - PubMed
LinkOut - more resources
Full Text Sources

