Vasoconstrictor antagonism improves functional and structural vascular alterations and liver damage in rats with early NAFLD
- PMID: 35036886
- PMCID: PMC8749167
- DOI: 10.1016/j.jhepr.2021.100412
Vasoconstrictor antagonism improves functional and structural vascular alterations and liver damage in rats with early NAFLD
Erratum in
-
Erratum Regarding Previously Published Articles.JHEP Rep. 2024 May 18;6(6):101097. doi: 10.1016/j.jhepr.2024.101097. eCollection 2024 Jun. JHEP Rep. 2024. PMID: 38978774 Free PMC article.
Abstract
Background & aims: Intrahepatic vascular resistance is increased in early non-alcoholic fatty liver disease (NAFLD), potentially leading to tissue hypoxia and triggering disease progression. Hepatic vascular hyperreactivity to vasoconstrictors has been identified as an underlying mechanism. This study investigates vasoconstrictive agonism and antagonism in 2 models of early NAFLD and in non-alcoholic steatohepatitis (NASH).
Methods: The effects of endothelin-1 (ET-1), angiotensin II (ATII) and thromboxane A2 (TxA2) agonism and antagonism were studied by in situ ex vivo liver perfusion and preventive/therapeutic treatment experiments in a methionine-choline-deficient diet model of steatosis. Furthermore, important results were validated in Zucker fatty rats after 4 or 8 weeks of high-fat high-fructose diet feeding. In vivo systemic and portal pressures, ex vivo transhepatic pressure gradients (THPG) and transaminase levels were measured. Liver tissue was harvested for structural and mRNA analysis.
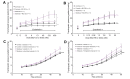
Results: The THPG and consequent portal pressure were significantly increased in both models of steatosis and in NASH. ET-1, ATII and TxA2 increased the THPG even further. Bosentan (ET-1 receptor antagonist), valsartan (ATII receptor blocker) and celecoxib (COX-2 inhibitor) attenuated or even normalised the increased THPG in steatosis. Simultaneously, bosentan and valsartan treatment improved transaminase levels. Moreover, bosentan was able to mitigate the degree of steatosis and restored the disrupted microvascular structure. Finally, beneficial vascular effects of bosentan endured in NASH.
Conclusions: Antagonism of vasoconstrictive mediators improves intrahepatic vascular function. Both ET-1 and ATII antagonists showed additional benefit and bosentan even mitigated steatosis and structural liver damage. In conclusion, vasoconstrictive antagonism is a potentially promising therapeutic option for the treatment of early NAFLD.
Lay summary: In non-alcoholic fatty liver disease (NAFLD), hepatic blood flow is impaired and the blood pressure in the liver blood vessels is increased as a result of an increased response of the liver vasculature to vasoconstrictors. Using drugs to block the constriction of the intrahepatic vasculature, the resistance of the liver blood vessels decreases and the increased portal pressure is reduced. Moreover, blocking the vasoconstrictive endothelin-1 pathway restored parenchymal architecture and reduced disease severity.
Keywords: ALT, alanine aminotransferase; ARB, angiotensin receptor blocker; AST, aspartate aminotransferase; ATII, angiotensin II; COX, cyclooxygenase; ET, endothelin; HFHFD, high-fat high-fructose diet; IHVR, intrahepatic vascular resistance; Jak2, Janus-kinase-2; MCD, methionine-choline deficient diet; Mx, methoxamine; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; NO, nitric oxide; PP, portal pressure; PR, pulse rate; SEM, scanning electron microscopy; TBW, total body weight; TEM, transmission electron microscopy; TXAS, thromboxane synthase; TxA2, thromboxane A2; ZFR, Zucker fatty rats; angiotensin II; endothelin-1; non-alcoholic fatty liver disease; portal hypertension; thromboxane A2; transhepatic pressure gradient.
© 2021 The Author(s).
Conflict of interest statement
Denise van der Graaff declares no conflict of interest. Shivani Chotkoe declares no conflict of interest. Benedicte De Winter declares no conflict of interest. Joris De Man declares no conflict of interest. Christophe Casteleyn declares no conflict of interest. Jean-Pierre Timmermans declares no conflict of interest. Isabel Pintelon declares no conflict of interest. Luisa Vonghia declares no conflict of interest. Wilhelmus J. Kwanten is co-inventor of a patent on the use lipopigment imaging for disease filed by MIT/MGH. Sven Francque has acted as advisor and/or lecturer for Roche, Gilead, Abbvie, Bayer, BMS, MSD, Janssen, Actelion, Astellas, Genfit, Inventiva, Intercept, Genentech, Galmed, Promethera, Coherus, NGM Bio and Julius Clinical. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures








References
-
- Van der Graaff D., Kwanten W.J., Francque S.M. The potential role of vascular alterations and subsequent impaired liver blood flow and hepatic hypoxia in the pathophysiology of non-alcoholic steatohepatitis. Med Hypoth. 2018;122:188–197. - PubMed
-
- Baffy G. Origins of portal hypertension in nonalcoholic fatty liver disease. Dig Dis Sci. 2018;63:563–576. - PubMed
-
- Francque S., Wamutu S., Chatterjee S., Van Marck E., Herman A., Ramon A., et al. Non-alcoholic steatohepatitis induces non-fibrosis-related portal hypertension associated with splanchnic vasodilation and signs of a hyperdynamic circulation in vitro and in vivo in a rat model. Liver Int. 2010;30:365–375. - PubMed
-
- Francque S., Verrijken A., Mertens I., Hubens G., Van Marck E., Pelckmans P., et al. Noncirrhotic human nonalcoholic fatty liver disease induces portal hypertension in relation to the histological degree of steatosis. Eur J Gastroenterol Hepatol. 2010;22:1449–1457. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

