Effect of Maternal Immunization With 10-Valent Pneumococcus Conjugate Vaccine (PCV-10), 23-Valent Pneumococcus Polysaccharide Vaccine, or Placebo on the Immunogenicity of PCV-10 in Human Immunodeficiency Virus-Exposed Uninfected Infants: A Randomized Clinical Trial
- PMID: 35037049
- PMCID: PMC10233289
- DOI: 10.1093/cid/ciac026
Effect of Maternal Immunization With 10-Valent Pneumococcus Conjugate Vaccine (PCV-10), 23-Valent Pneumococcus Polysaccharide Vaccine, or Placebo on the Immunogenicity of PCV-10 in Human Immunodeficiency Virus-Exposed Uninfected Infants: A Randomized Clinical Trial
Abstract
Background: The effect of pneumococcal vaccination of mothers with human immunodeficiency virus (HIV) on infant responses to childhood vaccination has not been studied. We compared the immunogenicity of 10-valent pneumococcus conjugate vaccine (PCV-10) in HIV-exposed uninfected infants born to mothers who received PCV-10, 23-valent pneumococcus polysaccharide vaccine (PPV-23), or placebo during pregnancy.
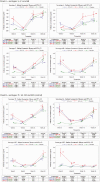
Methods: Antibody levels against 7 serotypes were measured at birth, before the first and second doses of PCV-10m and after completion of the 2-dose regimen in 347 infants, including 112 born to mothers who received PPV-23, 112 who received PCV-10, and 119 who received placebo during pregnancy. Seroprotection was defined by antibody levels ≥0.35 µg/mL.
Results: At birth and at 8 weeks of life, antibody levels were similar in infants born to PCV-10 or PPV-23 recipients and higher than in those born to placebo recipient. After the last dose of PCV-10, infants in the maternal PCV-10 group had significantly lower antibody levels against 5 serotypes than those in the maternal PPV-23 group and against 3 serotypes than those in the maternal placebo group, and they did not have higher antibody levels against any serotype. The seroprotection rate against 7 serotypes was 50% in infants in the maternal PCV-10 group, compared with 71% in both of the maternal PPV-23 and placebo groups (P < .001).
Conclusions: Administration of PCV-10 during pregnancy was associated with decreased antibody responses to PCV-10 and seroprotection rates in infants. Considering that PCV-10 and PPV-23 had similar immunogenicity in pregnant women with HIV and that administration of PPV-23 did not affect the immunogenicity of PCV-10 in infants, PPV-23 in pregnancy may be preferred over PCV-10.
Keywords: HIV infection; infant; maternal immunization; pneumococcal vaccine; vaccine immunogenicity.
© The Author(s) 2022. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Conflict of interest statement
Potential conflicts of interest. M. M. M. P. and G. D. report provision of PCV-10 by donation from Centro de Vigilância Epidemiológica da Secretaria da Saúde do Estado de São Paulo, São Paulo, Brazil. L. L. reports that Westat was the contract research organization contracted through the National Institutes of Health (NIH) for a task order for the current study. S. I. P. reports receipt of personal fees from Merck, Sanofi, and Pfizer; investigator-initiated grants from the NIH and Pfizer and Merck Vaccines to study surveillance of invasive pneumococcal disease, host defenses against Streptococcus pneumoniae, and the impact of severe acute respiratory syndrome coronavirus 2 on nasopharyngeal microbiome; and consulting fees from Pfizer and Merck Vaccine for participation in advisory board meetings, consulting on research projects at PAI Life Sciences and EVIDERA, and participation on a data safety and monitoring board for next-generation PCVs for Sanofi Pasteur. N. C. is employed by the NIH. A. W. has received grants from the NIH, GlaxoSmithKline, Janssen and Merck; consulting fees from Merck; and payment or honoraria from Merck. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures

References
-
- Troeger C, Blacker B, Khalil IA, et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis 2018; 18:1191–210. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

